Broca’s area, variation and taxic diversity in early Homo from Koobi Fora (Kenya)
Abstract
Because brain tissues rarely fossilize, pinpointing when and how modern human cerebral traits emerged in the hominin lineage is particularly challenging. The fragmentary nature of the fossil material, coupled with the difficulty of characterizing such a complex organ, has been the source of long-standing debates. Prominent among them are the uncertainties around the derived or primitive state of the brain organization in the earliest representatives of the genus Homo, more particularly in key regions such as the Broca’s area. By revisiting a particularly well-preserved fossil endocast from the Turkana basin (Kenya), here we confirm that early Homo in Africa had a primitive organization of the Broca’s area ca. 1.9 million years ago. Additionally, our description of KNM-ER 3732 adds further information about the variation pattern of the inferior frontal gyrus in fossil hominins, with implications for early Homo taxic diversity (i.e. one or two Homo species at Koobi Fora) and the nature of the mechanisms involved in the emergence of derived cerebral traits.
eLife assessment
This important study uses the brain endocast of a ~1.9-million-year-old hominin fossil from Kenya, attributed to genus Homo, to show that the organization of the Broca's area in members of early Homo was primitive. Specifically, the prefrontal sulcal pattern in this early Homo specimen more closely resembles that of chimpanzees than of modern humans. Because Broca's area is associated with speech function, the compelling evidence from this study is relevant for understanding the timing and trajectory of evolution of speech related traits in our genus. Coupled with its potential implications for taxonomic classification, this study will be of interest to paleoanthropologists, paleontologists, archaeologists, and neuroscientists.
https://doi.org/10.7554/eLife.89054.3.sa0Introduction
The modern human brain is an exceptionally complex, highly specialized, and extremely costly machinery. Because of the fragmentary nature of the hominin fossil record, assessing when and how changes in the brain of our ancestors happened, and inferring any related functional, behavioral, and metabolic consequences, is particularly challenging (Zollikofer and De León, 2013). Nonetheless, reconstructing the chronological and taxonomical context of the emergence of derived cerebral traits is a prerequisite for disentangling underlying evolutionary processes. For instance, local reorganization of specific areas in an overall primitive hominin brain would support a mosaic-like evolutionary pattern (e.g. Holloway et al., 2004), and raise essential questions on the role of selection pressure (or absence of; rev. in Beaudet, 2021). Beyond the value of such information on the origins of the human brain, the assumption that Homo developed a uniquely complex brain organization (e.g. Tobias, 1987) requires further evidence.
Although brains rarely fossilize, it is possible to glean structural information about the evolutionary history of the hominin brain by studying sulcal imprints in fossil brain endocasts (Neubauer, 2014). In this regard, the Broca’s area has been the focus of much interest in paleoneurology due to striking structural differences between extant human and chimpanzee brains and endocasts, and the implication of this area in articulated language (rev. in Beaudet, 2017). Ponce de León et al., 2021 thoroughly examined brain endocasts of Homo specimens in eastern Africa and Eurasia and demonstrated that the organization of the Broca’s area in the earliest representatives of the genus before 1.5 Ma was primitive. Because the imprints of the Broca’s cap in endocasts are not always readable, they used the coronal suture and surrounding sulcal imprints as a proxy to identify frontal lobe expansion (i.e. derived condition). Unfortunately, in some of the oldest and key specimens from Africa that could represent the >1.5 Ma condition (e.g. KNM-ER 1470) the interpretations remained inconclusive.
Testing the hypothesis of Ponce de León et al., 2021 of a primitive brain in the earliest representatives of the genus Homo before 1.5 Ma thus necessitates (i) an excellent preservation of very fine neuroanatomical details in fossil endocasts, and (ii) reliable information on their taxonomic identity (i.e. Homo) and stratigraphic context (i.e. before/after 1.5 Ma). The hominin specimen KNM-ER 3732 from Kenya, which fulfills these requirements, has the potential to shed new light on this conundrum. KNM-ER 3732 was discovered during the 1974–1975 field program in the Koobi Fora Formation (area 115), east of Lake Turkana in Kenya (Leakey, 1976; Wood, 1991). KNM-ER 3732 consists of a calotte, left zygoma, and a natural endocast. The expanded neurocranium and robust upper face support an attribution to Homo sp. indet. (Wood, 1991). The specimen was lying below the KBS Tuff of the upper Burgi Member that is dated to 1.87 million years ago, which provides a minimum age (Feibel et al., 1983; Feibel et al., 2006). Accordingly, if KNM-ER 3732 shows a derived cerebral condition, the hypothesis of >1.5 Ma Homo being associated with a primitive organization of the Broca’s area is falsified. Here, we provide a comparative study of the natural endocast of the pre-1.5 Ma Homo specimen KNM-ER 3732 to test the hypothesis of a late emergence of a modern Broca’s area in the hominin lineage.
Results
KNM-ER 3732 offers a glimpse of early Homo prefrontal organization
The natural endocast of KNM-ER 3732, initially described by Holloway et al., 2004, preserves the dorsal part but misses the frontal pole, the occipital and temporal lobes, as well as the entire ventral surface (Figure 1). Holloway et al., 2004 estimated the endocranial volume as about 750–800 cc but pointed out the low reliability of their estimate. Wood, 1991 (Page 132) identified an ‘irregular bony excrescence on the surface of the right parietal at the level of lambda and 32 mm from the midline. It projects 6 mm from the surface of the bone, and may be an example of myositis ossificans associated with damage to the overlying temporal muscle.’ Because of the location (right parietal) and nature (traumatic) of the outgrowth, it does not affect our area of study (i.e. left frontal bone) (Walczak et al., 2015). The gyral and sulcal details are well-preserved, particularly in the prefrontal region. The superior, middle, and inferior frontal sulci are visible on both hemispheres. On the left hemisphere, the Broca’s cap is prominent. A vertical groove, identified as the precentral sulcus, separates the Broca’s cap in half (Figure 2A). On both hemispheres, the central sulcus intersects the inter-hemispheric scissure and the post-central sulcus seems to be connected to the lateral fissure. The intra-parietal sulcus can be found in the parieto-occipital region of both hemispheres. The examination of the internal surface of the braincase (Figure 2B) confirms the pattern described on the natural endocast.
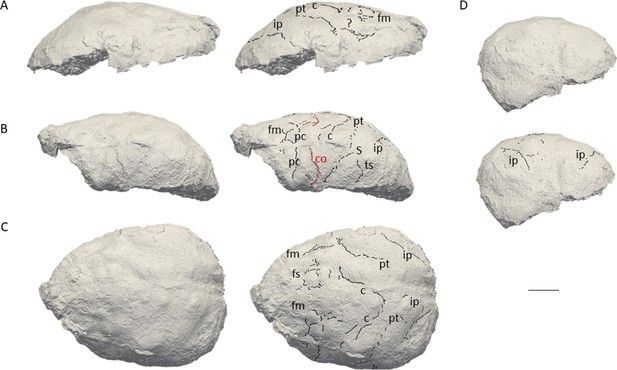
3D virtual rendering of the natural endocast of KNM-ER 3732 and identification of sulcal imprints.
KNM-ER 3732 is shown in the lateral right (A), lateral left (B), dorsal (C), and posterior (D) views. ar: ascending ramus of the lateral fissure; c: central sulcus; CO: coronal suture; fi: inferior frontal sulcus; fm: middle frontal sulcus; ip: intra-parietal sulcus; pc: pre-central sulcus; pt: post-central sulcus. Scale bar: 2 cm.
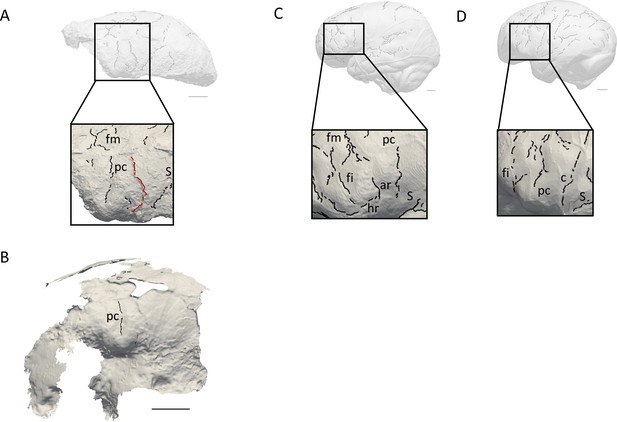
Comparison of the sulcal patterns identified in the inferior frontal area of KNM-ER 3732.
The natural endocast (A) and braincase (B) of KNM-ER 3732 are compared to the virtual endocasts of extant human (C) and chimpanzee (D) individuals. Images not to scale. ar: ascending ramus of the lateral fissure; c: central sulcus; co: coronal suture; fi: inferior frontal sulcus; fm: middle frontal sulcus; ip: intra-parietal sulcus; pc: pre-central sulcus; pt: post-central sulcus. Scale bar: 2 cm.
KNM-ER 3732 has a primitive prefrontal cortex
When compared to extant human and chimpanzee sulcal patterns, KNM-ER 3732 closely resembles the latter (Figure 2C–D). In extant human brains and endocasts, the inferior frontal sulcus often transects the Broca’s cap, while the ascending ramus of the lateral fissure caudally borders the prominence (Figure 2C–D; Connolly, 1950; de Jager et al., 2019; de Jager et al., 2022). In chimpanzees, the central sulcus is placed more rostrally, and the inferior portion of the precentral sulcus bisects the Broca’s cap that is bordered rostrally by the inferior frontal sulcus (Figure 2E–F; Connolly, 1950; Falk et al., 2018). The coronal suture runs in between the precentral and central sulci in KNM-ER 3732, which points towards the primitive configuration described in Ponce de León et al., 2021. The sulcal pattern seen in the prefrontal cortex of KNM-ER 3732, that was detected in both the natural endocast and the inner surface of the cranium (Figure 2A–B), and more particularly the deeply marked precentral sulcus that incised the Broca’s cap, is not found in any of the early Homo specimens described in Ponce de León et al., 2021 but approximates the condition seen in the Dmanisi cranium D2282 (Fig. S1B in Ponce de León et al., 2021). However, this pattern is seen in a contemporaneous non-Homo hominin specimen in South Africa, i.e. Australopithecus sediba (Falk, 2014).
Discussion
Paleoneuroanatomy supports taxic diversity within early Homo
Overall, the present study not only demonstrates that Ponce de León et al.’s (2021) hypothesis of a primitive brain of early Homo cannot be rejected, but also adds information about the variation pattern of the inferior frontal gyrus. In particular, the diversity of the prefrontal sulcal patterns of hominin endocasts at Koobi Fora revives the debate about the possible presence of two early Homo species in this locality (i.e. Homo habilis sensu stricto and Homo rudolfensis). In 1983, Falk, 1983 published the description of the endocasts of two Homo specimens from Koobi Fora, KNM-ER 1470 and KNM-ER 1805. Her analysis supported the co-existence of two morphs, KNM-ER 1470 representing a more derived human-like sulcal pattern. Interestingly, cerebral evidence brought up by her analysis matched other studies that emphasized the more derived craniodental anatomy of specimens attributed to Homo rudolfensis as opposed to the more primitive (Australopithecus-like) traits identified within Homo habilis sensu stricto (Leakey et al., 2012). While describing the external morphology of the neurocranium, (Leakey, 1976: 575) noted that KNM-ER 3732 was ‘strikingly similar to KNM-ER 1470.’ This resemblance is not reflected in their cerebral organization since the present study rather suggests a primitive organization of the Broca’s cap in KNM-ER 3732. If KNM-ER 1470 had indeed a derived brain, taxic diversity as a source of variation cannot be discarded. If we go further down that route, the similarities between KNM-ER 3732 and Australopithecus sediba suggested by our study could be an argument supporting the presence of Australopithecus in Koobi Fora or the absence of a definite threshold between the two genera based on the morphoarchitecture of their endocasts (Wood and Collard, 1999).
The evolutionary history of the human Broca’s area unraveled
Beyond the taxonomic aspect, variation detected in Koobi Fora could provide information about underlying evolutionary mechanisms, and more specifically the process of fixation of an adaptive variant, i.e. a new organization of the Broca’s area and the increase of neural interconnectivity in this region (Essen, 1997). Such neurological changes might have had deep implications for the emergence of novel behaviors, such as articulated language (Beaudet, 2017; Beaudet, 2021). Within this scenario, the study from Ponce de León et al., 2021 would suggest that this trait became stabilized by 1.5 million years ago. The identification of protracted brain growth as early as in Australopithecus afarensis (Gunz et al., 2020), inducing longer exposure to the social environment during brain maturation (rev. in Hublin et al., 2015), could be consistent with a derived organization of the Broca’s area being selected as a response to social environmental stimuli through developmental plasticity, culminating in this variant becoming dominant within Homo. In parallel, the possibility of allometric scaling and the influence of brain size on sulcal patterns in early Homo has to be further explored.
Materials and methods
Materials
KNM-ER 3732 is currently housed in the National Museums of Kenya in Nairobi (Kenya). We used brain and endocast atlases published in Connolly, 1950, Falk et al., 2018, and de Jager et al., 2019; de Jager et al., 2022; see also https://www.endomap.org/ for comparing the pattern identified in KNM-ER 3732 to those described in extant humans and chimpanzees. To the best of our knowledge, these atlases are the most extensive atlases of extant human and chimpanzee brains/endocasts available to date and are widely used in the literature to explore variability in sulcal patterns. In Figure 2, the extant human and chimpanzee conditions are illustrated by one extant human (adult female) and one extant chimpanzee (adult female) specimens from the Pretoria Bone Collection at the University of Pretoria (South Africa) and from the Royal Museum for Central Africa in Tervuren (Belgium), respectively (Beaudet et al., 2018).
Digitization
Request a detailed protocolBoth the natural endocast and the cranium of KNM-ER 3732 were scanned using an Artec Space Spider 3D scanner and reconstructed with the software Artec Studio 16 X. The 3D mesh can be viewed on MorphoSource. The comparative specimens were imaged by microfocus X-ray tomography (Beaudet et al., 2018) at the South African Nuclear Energy Corporation in Pelindaba (South Africa) and at the Centre for X-ray Tomography of Ghent University (UGCT) in Ghent (Belgium). Virtual endocasts were generated using Endex software (Subsol et al., 2010).
Detection and identification of sulcal imprints
Request a detailed protocolSulcal imprints were automatically detected through a geometry-based method using curvature lines computed from the natural endocast and the inner surface of the cranium. Sulcal imprints, considered as variation points of the surface on a triangle mesh, were detected through a geometry-based method using curvature lines defined as salient subsets of the extrema of the principal curvatures on surfaces (Yoshizawa et al., 2008; Beaudet et al., 2016). Vascular imprints and non-anatomical structures (e.g. fractures) were manually removed through a customized script written in MATLAB R2013a (Mathworks) that is available online (Dumoncel, 2019; https://gitlab.com/jeandumoncel/curve-editor).
Data availability
The 3D mesh of KNM-ER 3732 can be viewed on MorphoSource (https://www.morphosource.org/concern/media/000497752?locale=en). 3D models of comparative endocasts can be made available upon request if permission from the respective curators is granted. Indeed, extant human and chimpanzee endocasts derive from dry crania that are curated in osteological collections with controlled access. Ethics clearance must be obtained in the case of the human endocast. As such, an application should be submitted and approved by the curators. For the extant human endocast, permission should be requested from the Pretoria Bone Collection at the University of Pretoria, South Africa (Ericka L'Abbé, ericka.labbe@up.ac.za). For the extant chimpanzee endocasts, permission should be requested from the Royal Museum for Central Africa, Belgium (Emmanuel Gilissen, emmanuel.gilissen@africamuseum.be). If approved, the first author will share the 3D models of the endocasts. The customized script written in MATLAB R2013a (Mathworks) for editing detected curves is available online (https://gitlab.com/jeandumoncel/curve-editor).
References
-
Morphoarchitectural variation in South African fossil cercopithecoid endocastsJournal of Human Evolution 101:65–78.https://doi.org/10.1016/j.jhevol.2016.09.003
-
The Emergence of Language in the Hominin Lineage: perspectives from fossil endocastsFrontiers in Human Neuroscience 11:427.https://doi.org/10.3389/fnhum.2017.00427
-
Sulcal pattern variation in extant human endocastsJournal of Anatomy 235:803–810.https://doi.org/10.1111/joa.13030
-
Sulci 3D mapping from human cranial endocasts: A powerful tool to study hominin brain evolutionHuman Brain Mapping 43:4433–4443.https://doi.org/10.1002/hbm.25964
-
Interpreting sulci on hominin endocasts: old hypotheses and new findingsFrontiers in Human Neuroscience 8:134.https://doi.org/10.3389/fnhum.2014.00134
-
Stratigraphic context of fossil hominids from the Omo groups deposits: northern Turkana Basin, Kenya and EthiopiaAmerican Journal of Physical Anthropology 78:595–622.https://doi.org/10.1002/ajpa.1330780412
-
Stratigraphy, correlation, and age estimates for fossils from Area 123, Koobi ForaJournal of Human Evolution 57:112–122.https://doi.org/10.1016/j.jhevol.2009.05.007
-
Brain ontogeny and life history in Pleistocene homininsPhilosophical Transactions of the Royal Society B 370:20140062.https://doi.org/10.1098/rstb.2014.0062
-
Endocasts: possibilities and limitations for the interpretation of human brain evolutionBrain, Behavior and Evolution 84:117–134.https://doi.org/10.1159/000365276
-
3D automatic methods to segment “virtual” endocasts: state of the art and future directionsAmerican Journal of Physical Anthropology 141:226–227.
-
The brain of Homo habilis: A new level of organization in cerebral evolutionJournal of Human Evolution 16:741–761.https://doi.org/10.1016/0047-2484(87)90022-4
-
Myositis OssificansThe Journal of the American Academy of Orthopaedic Surgeons 23:612–622.https://doi.org/10.5435/JAAOS-D-14-00269
-
Fast, robust, and faithful methods for detecting crest lines on meshesComputer Aided Geometric Design 25:545–560.https://doi.org/10.1016/j.cagd.2008.06.008
-
Pandora’s growing box: Inferring the evolution and development of hominin brains from endocastsEvolutionary Anthropology 22:20–33.https://doi.org/10.1002/evan.21333
Peer review
Reviewer #1 (Public Review):
The cerebral cortex, or surface of the brain, is where humans do most of their conscious thinking. In humans, the grooves (sulci) and bumps (convolutions) have a particular pattern in a region of the frontal lobe called Broca's area, which is important for language. Specialists study features imprinted on the internal surfaces of braincases in early hominins by casting their interiors, which produces so-called endocasts. A major question about hominin brain evolution concerns when, where, and in which fossils a humanlike Broca's area first emerged, the answer to which may have implications for the emergence of language. The researchers used advanced imaging technology to study the endocast of a hominin (KNM-ER 3732) that lived about 1.9 million years ago (Ma) in Kenya to test a recently published hypothesis that Broca's remained primitive (apelike) prior to around 1.5 Ma. The results are consistent with the hypothesis and raise new questions about whether endocasts can be used to identify the genus and/or species of fossils.
https://doi.org/10.7554/eLife.89054.3.sa1Reviewer #2 (Public Review):
The authors present new data of endocranial surface details from the early Homo specimen KNM-ER 3732 and discuss the evolution of brain surface features that might be related to the evolution of language in the hominin lineage.
Comments and issues raised by the reviewers have been addressed adequately. I am sure that this contribution will revive discussion about these issues.
https://doi.org/10.7554/eLife.89054.3.sa2Reviewer #3 (Public Review):
The authors provide a detailed analysis of the sulcal and sutural imprints preserved on the natural endocast and associated cranial vault fragments of the KNM-ER3732 early Homo specimen. The analyses indicate a primitive ape-like organization of this specimen's frontal cortex. Given the geological age of around 1.9 million years, this is the earliest well-documented evidence of a primitive brain organization in African Homo.
The various points raised by the reviewers and the responses provided by the authors illustrate that paleoneurology is a research field where little consensus has been reached over the past century. This is due not only to the fragmentary preservation of most fossil endocasts, but also to the limitations of scientific inference in general, and paleoneurological inference in particular. Like any scientific hypothesis, a paleoneurological hypothesis cannot be proven, but at best be falsified, leaving a wide field of possible alternative hypotheses. Furthermore, endocranial morphology does not equate cerebral morphology. A classical example: the endocranial Broca cap is not identical to the cortical Broca area. And last but not least, taxonomy cannot resolve questions of phylogeny.
https://doi.org/10.7554/eLife.89054.3.sa3Author response
The following is the authors’ response to the original reviews.
Reviewer #1 (Public Review):
The cerebral cortex, or surface of the brain, is where humans do most of their conscious thinking. In humans, the grooves (sulci) and bumps (convolutions) have a particular pattern in a region of the frontal lobe called Broca's area, which is important for language. Specialists study features imprinted on the internal surfaces of braincases in early hominins by casting their interiors, which produces so-called endocasts. A major question about hominin brain evolution concerns when, where, and in which fossils a humanlike Broca's area first emerged, the answer to which may have implications for the emergence of language. The researchers used advanced imaging technology to study the endocast of a hominin (KNM-ER 3732) that lived about 1.9 million years ago (Ma) in Kenya to test a recently published hypothesis that Broca's remained primitive (apelike) prior to around 1.5 Ma. The results are consistent with the hypothesis and raise new questions about whether endocasts can be used to identify the genus and/or species of fossils.
We would like to thank Rev. 1 for their comments on our paper.
Reviewer #2 (Public Review):
The authors tried to support the hypothesis that early Homo still had a primitive condition of Broca's cap (the region in fossil endocasts corresponding to Broca's area in the brain), being more similar to the condition in chimpanzees than in humans. The evidence from the described individual points to this direction but there are some flaws in the argumentation.
We are grateful to Rev. 2 for their comments, although we partially agree with some of them.
First, we would like to rectify the statement of Rev. 2 that we “tried to support the hypothesis that early Homo still had a primitive condition of Broca's cap”, indeed, our aim was to test this hypothesis and not to try to validate it.
First, only one human and one chimpanzee were used for comparison, although we know that patterns of brain convolutions (and in addition how they leave imprints in the endocranial bones) are very variable.
We understand the point raised by Rev. 2 about the variation of brain convolutions in humans and chimpanzees. We used atlases published by Connolly (1950), Falk et al. (2018) and de Jager et al. (2019, 2022) to analyse the endocast of KNM-ER 3732 and compare it to the extant human and chimpanzee cerebral conditions. However, in Figure 2, for the sake of clarity only two Homo and Pan specimens were used to illustrate the comparison (as it has been done in other published papers, e.g., Carlson et al., 2011; Science, Gunz et al., 2020 Sci Adv). In the revised version, we modified the manuscript to explain further our approach (line 156) “We used brain and endocast atlases published in Connolly (1950), Falk et al. (2018) and de Jager et al. (2019, 2022; see also www.endomap.org) for comparing the pattern identified in KNM-ER 3732 to those described in extant humans and chimpanzees. To the best of our knowledge, these atlases are the most extensive atlases of extant human and chimpanzee brains/endocasts available to date and are widely used in the literature to explore variability in sulcal patterns. In Figure 2, the extant human and chimpanzee conditions are illustrated by one extant human (adult female) and one extant chimpanzee (adult female) specimens from the Pretoria Bone Collection at the University of Pretoria (South Africa) and in the Royal Museum for Central Africa in Tervuren (Belgium), respectively (Beaudet et al., 2018).”.
Second, the evidence from this fossil specimen adds to the evidence of previously describe individuals but still not yet fully prove the hypothesis.
We tempered our discussion by concluding that (line 116) “Overall, the present study not only demonstrates that Ponce de León et al.’s (2021) hypothesis of a primitive brain of early Homo cannot be rejected, but also adds information […]”.
Third, there is a vicious circle in using primitive and derived features to define a fossil species and then using (the same or different) features to argue that one feature is primitive or derived in a given species. In this case, we expect members of early Homo to be derived compared to their predecessors of the genus Australopithecus and that's why it seems intriguing and/or surprising to argue that early Homo has primitive features. However, we should expect that there is some kind of continuum or mosaic in a time in which a genus "evolves into" another genus. This discussion requires far more discussions about the concepts we use, maybe less discussion about what is different between the two groups but more discussion about the evolutionary processes behind them.
We fully agree with Rev. 2 on this aspect. We believe that identifying these differences/similarities between fossil and extant hominids constitute the first step of a better understanding of the evolutionary mechanisms. Our work suggests indeed a certain continuity between genera and raises questions on the genus concept and how to interpret the specimens currently attributed to early Homo. In the revised version of the manuscript we included a reference to this possible scenario (line 134): “[…] or to the absence of a definite threshold between the two genera based on the morphoarchitecture of their endocasts (Wood and Collard, 1999).”.
Fourth, the data of convolutional imprints presented are rather subjective when identifying which impressions represent which brain convolutions. Not seeing an impression does not necessarily mean that the corresponding brain feature did not exist. Interestingly, the manuscript does not mention and discuss at all the frontoorbital sulcus. This is a sulcus that usually runs from the orbital surface of the frontal lobe up to divide the inferior frontal gyrus in chimpanzees, a condition totally different than in humans who do not have a frontoorbital sulcus. Could such a sulcus be identified, this would provide a far more convincing argument for a primitive condition in this specimen. In Australopithecus sediba, e.g., the condition in this region seems to be a mosaic in which some aspects of the morphology seem to be more modern while one of the sulcual impressions can well be interpreted as a short frontoorbital sulcus. For this specimen, by the way, I would come back to my third point above: some experts in the field might argue that this specimen could belong to Homo rather than Australopithecus...
We agree that the presence of a fronto-orbital sulcus would be more conclusive. However, this sulcus has not been identified in KNM-ER3732 and the region in which we would expect to find it is not preserved. As demonstrated by Ponce de León et al. (2021), because of the topographic relationships between sulci (and cranial structures), it is possible to interpret imprints on endocasts and the evolutionary polarity of some traits even in the absence of landmarks such as the fronto-orbital sulcus. In Australopithecus sediba the main derived feature of the endocast corresponds to the ventrolateral bulge in the left inferior frontal gyrus, and not to the sulcal pattern itself (Carlson et al., 2011 Science). However, the discussion around the taxonomic status of this taxon confirms the urgent need for reconsidering specimens from that time period and clarifying the mosaic-like or concerted evolution of the derived Homo-like traits within our lineage. Regarding the subjective nature of this approach, we invite readers to examine the specimen on MorphoSource (https://www.morphosource.org/concern/media/000497752?locale=en) and to request access to the National Museums of Kenya to the physical or virtual specimen to falsify our hypothesis.
According to my arguments above, I think that this manuscript might revive interesting discussions about this topic but it is not likely to settle them because the data presented are not strong enough to fully support the hypothesis.
We would be more than happy to consider new/other specimens with similar chronological and geographical contexts and investigate further this hypothesis in the future.
Reviewer #3 (Public Review):
The authors provide a detailed analysis of the sulcal and sutural imprints preserved on the natural endocast and associated cranial vault fragments of the KNM-ER3732 early Homo specimen. The analyses indicate a primitive ape-like organization of this specimen's frontal cortex. Given the geological age of around 1.9 million years, this is the earliest well-documented evidence of a primitive brain organization in African Homo.
In the discussion, the authors re-assess one of the central questions regarding the evolution of early Homo: was there species diversity, and if yes, how can we ascertain it? The specimen KNM-ER1470 has assumed a central role in this debate because it purportedly shows a more advanced organization of the frontal cortex compared to other largely coeval specimens (Falk, 1983). However, as outlined in Ponce de León et al. 2021 (Supplementary Materials), the imprints on the ER1470 endocranium are unlikely to represent sulcal structures and are more likely to reflect taphonomic fracturing and distortion. Dean Falk, the author of the 1983 study, basically shares this view (personal communication). Overall, I agree with the authors that the hypothesis to be tested is the following: did early Homo populations with primitive versus derived frontal lobe organizations coexist in Africa, and did they represent distinct species?
I greatly appreciate that the authors make available the 3D surface data of this interesting endocast.
We are grateful to Rev. 3 for their comments and for contextualizing our finding. We would also like to point out that, although the 3D surface can be viewed on MorphoSource, permission from the National Museums of Kenya has to be requested for studying the specimen and getting access to the physical specimen and/or the 3D model.
Reviewer #1 (Recommendations For The Authors):
Holloway, Broadfield & Yuan (2004) estimate ER 3732 as having a cranial capacity of 750 cc, which is larger than chimps and australopiths and similar to ER 1470 (752 cc, same reference). (That for Dmanisi 2282 is somewhat smaller at around 650 cc.) Cranial capacities should be mentioned along with added discussion about possible allometric scaling of (increased) numbers of sulci with increasing brain size as well as possible shifts in locations of sulci relative to cranial sutures in larger-brained (including due to ontogenetic maturation) in individuals/species. Could these variables (especially brain size) be relevant for your discussion/conclusions?
We thank Rev. 1 for their suggestion. We included the estimate by Holloway et al. (2004) (line 95): “Holloway et al. (2004) estimated the endocranial volume as about 750-800 cc but insisted on the low reliability of their estimate.”. Additionally, we raised the possibility of potential allometric effect (line 149): “In parallel, the possibility of allometric scaling and influence of brain size on sulcal patterns in early Homo has to be further explored.” for future discussion.
From the two figures, it appears that the authors produced a virtual endocast from the cranial remains of ER 3732 and compared its features with those seen on a virtual reproduction of the corresponding natural endocast. If so, this needs to be clarified in the text, not just the figures.
We thank Rev. 1 for their suggestions that were integrated.
Reviewer #3 (Recommendations For The Authors):
While the sulcal imprints on the left hemisphere can be interpreted unambiguously, the anatomical assignment of those on the right side may need to be reconsidered, as they are more ambiguous. For example, the postcentral sulcus (pt) almost touches the middle frontal sulcus, which is an unlikely natural configuration.
We agree that the configuration on the right hemisphere is intriguing, especially when compared to the extant human and chimpanzee atlases. As such, we decided to change the label for what we think could be the inferior frontal sulcus and leave a question mark instead.
I encourage the authors to include:
A posterior view in Figure 1, and mark the lambdoid suture, parts of which seem to be preserved especially on the left side. This will help the readership to better understand which parts of the endocranial morphology are preserved.
A scale bar would be of great utility to appreciate the small size of this specimen. The distance from bregma to the Broca cap seems to be short, indicating an endocranial volume much smaller than the published estimate of 750 ccm. Perhaps the authors can provide a new estimate, which would provide further support for the arguments proposed in the discussion section, especially the question of any presence of Australopithecus at Koobi Fora.
We included a posterior view of the specimen in Figure 1 and scale bar and modified the legend accordingly. Unfortunately, we were not able to identify with certainty the feature that could correspond to the lambdoid suture. We might see the impression where the parietal bone meets the occipital bone, but there is a risk of misidentification (which is an issue frequently raised in the literature, see for example Gunz et al. 2020 Sci Adv). Concerning the endocranial volume, in the revised version of the manuscript we included the estimate by Holloway et al. (2004). Because the specimen only preserves the superior part, we are reluctant in providing an estimate of the total volume. However, we agree that this would be an interesting feature to integrate in the interpretation of this specimen.
Minor points
This sentence needs to be clarified: «The superior temporal sulcus nearly intersects the lateral fissure on the right hemisphere».
The terms «Broca's region» and «orbital cap» need some more context. Do the authors mean «Broca's cap» in either instance?
We clarified/modified when needed, thank you very much.
We included minor corrections in addition to those recommended by the reviewers:
-Lines 50, 74, 142, 149: “Broca’s area” instead of “Broca’s cap”
-Line 73: “in the pre-1.5 Ma Homo specimen” instead of “in pre-1.5 Ma Homo specimen”
-Line 100: we specified “in human brains and endocasts”
-Line 120: “sulcal pattern” instead of “sulcal patterns”
-Line 144: “behaviors” (plural)
https://doi.org/10.7554/eLife.89054.3.sa4Article and author information
Author details
Funding
National Research Foundation (129336)
- Amélie Beaudet
University of Cambridge
- Amélie Beaudet
Centre National de la Recherche Scientifique (CPJ-Hominines)
- Amélie Beaudet
University of Cambridge (Harding Distinguished Postgraduate Scholars Programme)
- Edwin de Jager
South Africa/France Joint Research Programme (129923)
- Amélie Beaudet
McDonald Institute for Archaeological Research
- Amélie Beaudet
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank Emmanuel Ndiema and the curatorial staff of the National Museums of Kenya for collection access. For scientific/technical discussion, we are grateful to: G Castelli (Cambridge), M Mirazón‐Lahr (Cambridge), R Holloway (New York), F Spoor (London). AB is funded by the National Research Foundation of South Africa (Research Development Grants for Y-Rated Researchers, grant number 129336), the University of Cambridge, and the Centre National de la Recherche Scientifique. EdJ is funded by the University of Cambridge Harding Distinguished Postgraduate Scholars Programme. AB and EdJ are funded by the South Africa/France (PROTEA) Joint Research Programme (grant number 129923) and the McDonald Institute for Archaeological Research. We are grateful to the three reviewers and the editors for their comments and suggestions.
Ethics
Ethical clearance for the use of extant human cranium was obtained from the Main Research Ethics committee of the Faculty of Health Sciences, University of Pretoria in February 2016.
Senior Editor
- George H Perry, Pennsylvania State University, United States
Reviewing Editor
- Yonatan Sahle, University of Cape Town, South Africa
Version history
- Sent for peer review: May 10, 2023
- Preprint posted: June 7, 2023 (view preprint)
- Preprint posted: June 30, 2023 (view preprint)
- Preprint posted: September 4, 2023 (view preprint)
- Version of Record published: September 18, 2023 (version 1)
Cite all versions
You can cite all versions using the DOI https://doi.org/10.7554/eLife.89054. This DOI represents all versions, and will always resolve to the latest one.
Copyright
© 2023, Beaudet and de Jager
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 401
- Page views
-
- 55
- Downloads
-
- 0
- Citations
Article citation count generated by polling the highest count across the following sources: Crossref, PubMed Central, Scopus.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Evolutionary Biology
- Genetics and Genomics
Microbial plankton play a central role in marine biogeochemical cycles, but the timing in which abundant lineages diversified into ocean environments remains unclear. Here, we reconstructed the timeline in which major clades of bacteria and archaea colonized the ocean using a high-resolution benchmarked phylogenetic tree that allows for simultaneous and direct comparison of the ages of multiple divergent lineages. Our findings show that the diversification of the most prevalent marine clades spans throughout a period of 2.2 Ga, with most clades colonizing the ocean during the last 800 million years. The oldest clades – SAR202, SAR324, Ca. Marinimicrobia, and Marine Group II – diversified around the time of the Great Oxidation Event, during which oxygen concentration increased but remained at microaerophilic levels throughout the Mid-Proterozoic, consistent with the prevalence of some clades within these groups in oxygen minimum zones today. We found the diversification of the prevalent heterotrophic marine clades SAR11, SAR116, SAR92, SAR86, and Roseobacter as well as the Marine Group I to occur near to the Neoproterozoic Oxygenation Event (0.8–0.4 Ga). The diversification of these clades is concomitant with an overall increase of oxygen and nutrients in the ocean at this time, as well as the diversification of eukaryotic algae, consistent with the previous hypothesis that the diversification of heterotrophic bacteria is linked to the emergence of large eukaryotic phytoplankton. The youngest clades correspond to the widespread phototrophic clades Prochlorococcus, Synechococcus, and Crocosphaera, whose diversification happened after the Phanerozoic Oxidation Event (0.45–0.4 Ga), in which oxygen concentrations had already reached their modern levels in the atmosphere and the ocean. Our work clarifies the timing at which abundant lineages of bacteria and archaea colonized the ocean, thereby providing key insights into the evolutionary history of lineages that comprise the majority of prokaryotic biomass in the modern ocean.
-
- Evolutionary Biology
- Genetics and Genomics
In many species, meiotic recombination events tend to occur in narrow intervals of the genome, known as hotspots. In humans and mice, double strand break (DSB) hotspot locations are determined by the DNA-binding specificity of the zinc finger array of the PRDM9 protein, which is rapidly evolving at residues in contact with DNA. Previous models explained this rapid evolution in terms of the need to restore PRDM9 binding sites lost to gene conversion over time, under the assumption that more PRDM9 binding always leads to more DSBs. This assumption, however, does not align with current evidence. Recent experimental work indicates that PRDM9 binding on both homologs facilitates DSB repair, and that the absence of sufficient symmetric binding disrupts meiosis. We therefore consider an alternative hypothesis: that rapid PRDM9 evolution is driven by the need to restore symmetric binding because of its role in coupling DSB formation and efficient repair. To this end, we model the evolution of PRDM9 from first principles: from its binding dynamics to the population genetic processes that govern the evolution of the zinc finger array and its binding sites. We show that the loss of a small number of strong binding sites leads to the use of a greater number of weaker ones, resulting in a sharp reduction in symmetric binding and favoring new PRDM9 alleles that restore the use of a smaller set of strong binding sites. This decrease, in turn, drives rapid PRDM9 evolutionary turnover. Our results therefore suggest that the advantage of new PRDM9 alleles is in limiting the number of binding sites used effectively, rather than in increasing net PRDM9 binding. By extension, our model suggests that the evolutionary advantage of hotspots may have been to increase the efficiency of DSB repair and/or homolog pairing.






