The myocardium utilizes a platelet-derived growth factor receptor alpha (Pdgfra)–phosphoinositide 3-kinase (PI3K) signaling cascade to steer toward the midline during zebrafish heart tube formation
Abstract
Coordinated cell movement is a fundamental process in organ formation. During heart development, bilateral myocardial precursors collectively move toward the midline (cardiac fusion) to form the primitive heart tube. Extrinsic influences such as the adjacent anterior endoderm are known to be required for cardiac fusion. We previously showed however, that the platelet-derived growth factor receptor alpha (Pdgfra) is also required for cardiac fusion (Bloomekatz et al., 2017). Nevertheless, an intrinsic mechanism that regulates myocardial movement has not been elucidated. Here, we show that the phosphoinositide 3-kinase (PI3K) intracellular signaling pathway has an essential intrinsic role in the myocardium directing movement toward the midline. In vivo imaging further reveals midline-oriented dynamic myocardial membrane protrusions that become unpolarized in PI3K-inhibited zebrafish embryos where myocardial movements are misdirected and slower. Moreover, we find that PI3K activity is dependent on and interacts with Pdgfra to regulate myocardial movement. Together our findings reveal an intrinsic myocardial steering mechanism that responds to extrinsic cues during the initiation of cardiac development.
Editor's evaluation
This is a valuable study that shows the involvement of phosphoinositide 3-kinase (PI3K) signaling downstream of platelet-derived growth factor receptor α in latero-medial migration of cardiomyocytes during the formation of the early heart tube during zebrafish development. The authors provide convincing evidence for the role of PI3K in cardiomyocyte migration using multiple PI3K inhibitory drugs, expression of a dominant negative PI3K subunit, and rescue of the Pdgfaa ligand over-expression phenotype using mild PI3K inhibition, approaches which show strong alignment and which are quantified using live imaging. The demonstration of cardiomyocyte protrusions biased in the direction of migration, and randomised after PI3K inhibition, is a promising area for future exploration.
https://doi.org/10.7554/eLife.85930.sa0Introduction
During organogenesis, cell progenitor populations often need to move from their origin of specification to a new location in order to form a functional organ. Deficient or inappropriate movement can underlie congenital defects and disease. Directing these movements can involve extrinsic factors such as chemical and mechanical cues from neighboring tissues and the local environment as well as intrinsic mechanisms such as intracellular signaling and polarized protrusions (Schumacher, 2019). Progenitor cell movement occurs during cardiac development, where myocardial cells are specified bilaterally on either side of the embryo (Stainier et al., 1993). To form a single heart that is centrally located, these bilateral populations must move to the midline and merge (Wilens, 1955; Rawles, 1936). As they move, myocardial cells undergo a mesenchymal-to-epithelial (MET) transition forming intercellular junctions and subsequently moving together as an epithelial collective (Trinh and Stainier, 2004; Jackson et al., 2017; Dominguez et al., 2023; Linask, 1992; Holtzman et al., 2007). This process is known as cardiac fusion and occurs in all vertebrates (Davidson et al., 2005; Evans et al., 2010).
External influence from the adjacent endoderm is essential for the collective movement of myocardial cells toward the midline. Mutations in zebrafish and mice which inhibit endoderm specification or disrupt endoderm morphogenesis result in cardia bifida – a phenotype in which the bilateral myocardial populations fail to merge (Holtzman et al., 2007; Alexander et al., 1999; Kupperman et al., 2000; Osborne et al., 2008; Kawahara et al., 2009; Kikuchi et al., 2001; Mendelson et al., 2015; Molkentin et al., 1997; Ye and Lin, 2013; Li et al., 2004; Yelon et al., 1999). Similar phenotypes also occur in chicks and rats when the endoderm is mechanically disrupted (Goss, 1935; Rosenquist, 1970; Varner and Taber, 2012). Studies simultaneously observing endoderm and myocardial movement have found a correlation between the movements of these two tissues, suggesting a model in which the endoderm provides the mechanical force that pulls myocardial cells toward the midline (Varner and Taber, 2012; Cui et al., 2009; Ye et al., 2015; Aleksandrova et al., 2015). Yet, these correlations do not occur at all stages of cardiac fusion, indicating that myocardial cells may also use intrinsic mechanisms to actively move toward the midline. Indeed, recent studies revealing a role for the receptor tyrosine kinase, platelet-derived growth factor receptor alpha (Pdgfra) in the movement of myocardial cells have suggested a paracrine chemotaxis model, in which the myocardium senses chemokine signals from the endoderm and responds to them (Bloomekatz et al., 2017). However, the existence and identity of these intrinsic myocardial mechanisms remain to be fully elucidated.
We have sought to identify the intracellular pathways downstream of Pdgfra that regulate the collective movement of the myocardium. The phosphoinositide 3-kinase (PI3K) pathway is known as an intracellular signaling mediator of receptor tyrosine kinases (RTKs; e.g. Pdgfra). During PI3K signaling, PI3K phosphorylates phosphatidylinositol (4,5)-bisphosphate (PIP2) to create phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which recruits pleckstrin homology (PH)-domain containing proteins and regulates many cellular processes including proliferation and cell migration (Fruman et al., 2017). Both individualistic cell migration such as in Dictyostelium and neutrophils (Iijima and Devreotes, 2002; Yoo et al., 2010) and collective cell migration such as in the movement of border cells in Drosophila and the movement of the anterior visceral endoderm during mouse gastrulation (Ghiglione et al., 2018; Bloomekatz et al., 2012) have been shown to be regulated by PI3K signaling.
Using the advantages of external development and ease of live imaging in the zebrafish model system (Shrestha et al., 2020), our studies reveal that myocardial PI3K signaling is required for proper directional movement toward the midline during cardiac fusion. In particular, we find that inhibition of the PI3K pathway, throughout the embryo or only in the myocardium, results in bilateral cardiomyocyte populations that fail to reach the midline (cardia bifida) or have only partially merged by the time wild-type myocardial cells are fully merged. High-resolution live imaging in combination with mosaic labeling further reveals that the orientation of myocardial membrane protrusions during cardiac fusion is dependent on PI3K signaling. Furthermore, we find that PI3K and Pdgfra interact to facilitate cardiac fusion. Altogether our work supports a model by which intrinsic Pdgfra–PI3K signaling regulates the formation of membrane protrusions, facilitating the collective movement of the myocardium toward the midline. Insight into the balance of extrinsic and intrinsic influences for directing collective movement of myocardial cells has implications for understanding a wide set of congenital and environmental cardiac defects as well as the pathogenic mechanisms of diseases broadly associated with collective movement.
Results
The PI3K pathway is required for proper cardiac fusion
In a search for intracellular signaling pathways that are important for cardiac fusion, we examined the PI3K signaling pathway by pharmacological inhibition of PI3K activity with LY294002 (LY) (Vlahos et al., 1994). Treatments were started at bud stage (10 hours post-fertilization – hpf), in order to exclude effects on mesodermal cells during gastrulation (Montero et al., 2003). In wild-type or dimethyl sulfoxide (DMSO)-treated embryos, bilateral myocardial populations move toward the midline and merge to form a ring structure between 20 and 21 hpf, which corresponds to the 20–22 somite stage (s) (Figure 1A, A', F, G). However, in embryos treated with 15–25 μM LY myocardial movement is disrupted and the bilateral myocardial populations fail to properly merge by 22s (Figure 1B, B', F, G, Figure 1—figure supplement 1A–C, M). To ensure our analysis of cardiac fusion phenotypes was not complicated by a developmental delay, we used developmentally stage-matched embryos (somite stage) rather than time-matched embryos (hpf) (see Figure 1—figure supplement 2 for an analysis using time-matched embryos).
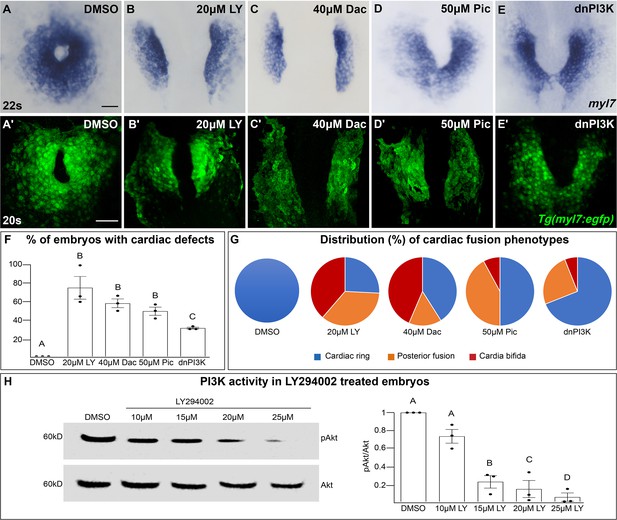
The phosphoinositide 3-kinase (PI3K) pathway is required for cardiac fusion.
Dorsal views, anterior to the top, of the myocardium labeled with myl7 (A–E) at 22 somite stage (s) or Tg(myl7:egfp) (A'–E') at 20s. In contrast to a ring of myocardial cells in DMSO-treated embryos (A, A'), in embryos treated with PI3K inhibitors LY294002 (LY, B, B'), Dactolisib (Dac, C, C'), or Pictilisib (Pic, D, D') at bud stage or injected with dnPI3K mRNA (750 pg) at the one-cell stage (E, E') cardiac fusion fails to occur properly with embryos displaying either cardia bifida (B, C) or fusion only at the posterior end (D, E). Graphs depict the percentage (F) and range (G) of cardiac fusion defects in control and PI3K-inhibited embryos. Dots represent the percent of embryos with cardiac defects per biological replicate. Total embryos analyzed n = 37 (DMSO), 31 (20 μM LY), 39 (40 μM Dac), 38 (50 μM Pic), and 86 (dnPI3K). Blue – cardiac ring/normal; orange – fusion only at posterior end/mild phenotype, red – cardia bifida/severe phenotype. (H) Representative immunoblot and ratiometric analysis of phosphorylated Akt (pAkt) to Akt protein levels in DMSO- and LY-treated embryos reveals a dose-dependent decrease in PI3K activation. Bar graphs indicate mean ± standard error of the mean (SEM), dots indicate pAKT/AKT ratio per biological replicate, normalized to DMSO. Three biological replicates per treatment. One-way analysis of variance (ANOVA) tests – letter changes indicate differences of p < 0.05 (F, H). Scale bars, 40 μm (A–E), 42 μm (A'–E'). Raw data and full p-values included in the source file.
-
Figure 1—source data 1
Statistical source data for Figure 1F, H.
- https://cdn.elifesciences.org/articles/85930/elife-85930-fig1-data1-v3.xlsx
-
Figure 1—source data 2
Original immunoblots used in Figure 1H (raw, uncropped) with and without labeling.
- https://cdn.elifesciences.org/articles/85930/elife-85930-fig1-data2-v3.zip
LY targets class I PI3K complexes, which is useful since class I complexes are known to be functionally redundant (Jean and Kiger, 2014; Juss et al., 2012). However, at higher concentrations than required for inhibiting class I PI3K complexes, LY has also been shown to inhibit non-PI3K complexes (off-targets) (Gharbi et al., 2007). To exclude the possibility that LY-induced cardiac fusion phenotypes result from off-target artifacts, we exposed bud stage embryos to two other PI3K inhibitors, Dactolisib (Dac) or Pictilisib (Pic) (Raynaud et al., 2009; Folkes et al., 2008; Maira et al., 2008). Exposure to these PI3K inhibitors (20–50 μM) causes cardiac fusion defects (Figure 1A–D, A'–D', F, G, Figure 1—figure supplement 1D–I, N, O) and corresponding reductions in PI3K activity, as measured by the ratio of phosphorylated AKT (pAKT) to AKT (Figure 1H, Figure 1—figure supplement 1Q–R). AKT is phosphorylated as a direct consequence of PI3K activity (Alessi et al., 1996). Despite the increased specificity of Dac and Pic, all three inhibitors (Dac, Pic, and LY) have been shown to also inhibit mTOR (Folkes et al., 2008; Maira et al., 2008; Brunn et al., 1996), as well as class I PI3K complexes. Thus, we directly examined a role for mTOR in cardiac fusion. Incubating embryos from bud stage to 22s with rapamycin, an inhibitor of mTOR (Heitman et al., 1991), at multiple concentrations did not affect cardiac fusion (Figure 1—figure supplement 3A–F), even though mTOR activity as measured by S6 phosphorylation (Holz and Blenis, 2005) was dramatically reduced (Figure 1—figure supplement 3G). Furthermore, using a non-pharmacological approach we found that mRNA injection of a truncated form of p85, which acts as a dominant negative inhibitor of PI3K (dnPI3K) activity (Carballada et al., 2001) also caused cardiac fusion defects (Figure 1E, E', F, G, Figure 1—figure supplement 1J–L, P, S). Thus, inhibition of PI3K activity with LY, Dac, Pic, and dominant-negative p85 mRNA, but not mTOR causes cardiac fusion defects.
We next characterized the morphology of the cardiac ring in PI3K-inhibited embryos and the cellular processes known to be regulated by PI3K signaling. During the later stages of cardiac fusion, myocardial cells develop epithelial polarity in which proteins such as ZO1 form intercellular junctions between myocardial cells (Trinh and Stainier, 2004; Jackson et al., 2017). During this time, the myocardium also moves deeper into the tissue eventually residing ventral to the endoderm in a process known as subduction (Ye et al., 2015) and it forms a contiguous second dorsal layer (Figure 1—figure supplement 4A–C, arrows). In PI3K-inhibited embryos, we found that myocardial cells form this second dorsal layer however, the localization of polarity markers and the tissue organization can appear mildly disorganized (Figure 1—figure supplement 4D–F). The PI3K signaling pathway is known to promote cell proliferation and cell survival (Fruman et al., 2017) however, we did not find a difference in the number of cardiomyocytes (Figure 1—figure supplement 4G–I) nor in the number of cardiomyocytes in S-phase as measured by EdU incorporation (Figure 1—figure supplement 4J–L) in PI3K-inhibited embryos at 20s compared to DMSO-treated embryos. Similarly, no apoptotic cardiomyocytes were observed in DMSO- nor in 20 μM LY-treated embryos (Figure 1—figure supplement 4M–P, n = 17, 19 embryos, respectively, from three biological replicates), despite apoptosis being observed in DNAse-treated controls (Figure 1—figure supplement 4O, P).
We further investigated whether increasing PI3K signaling affects cardiac fusion, by examining ptena, ptenb mutants. Pten, a lipid phosphatase, opposes PI3K function by converting PIP3 to PIP2 (Fruman et al., 2017). Homozygous ptena−/− mutants, along with double homozygous ptena−/−, ptenb−/− mutants did not show defects in cardiac fusion (Figure 1—figure supplement 5A–C, J). However, maternal contribution of Pten has been reported to persist till at least 60 hpf (Choorapoikayil et al., 2013; Faucherre et al., 2008), possibly explaining the lack of phenotype in these mutants. Indeed, cardiac fusion defects have been observed in pten mutant mouse embryos (Bloomekatz et al., 2012) and we found that inhibiting Pten in zebrafish embryos from bud stage to 22s with the small molecule Pten inhibitor VO-OHpic (Rosivatz et al., 2006) does result in cardiac fusion defects, along with increased PI3K activity (Figure 1—figure supplement 6). Furthermore, adding a sub-phenotypic dose of VO-OHpic (Figure 1—figure supplement 5D–F) to double homozygous ptena−/−, ptenb−/− mutant embryos to address the role of the remaining maternal contribution also results in cardiac fusion defects (Figure 1—figure supplement 5G–J, Fisher’s test p-value 8.21E−06). These findings reveal that loss-of-Pten which causes increased PIP3 levels results in cardiac fusion defects and together with our PI3K inhibition experiments indicate that appropriate levels of PI3K signaling are required for proper cardiac fusion.
The extent and duration of PI3K inhibition determine the penetrance and severity of cardiac fusion defects
PI3K-inhibited embryos display cardiac phenotypes at 22s that range from severe, in which the myocardial populations remain entirely separate (cardia bifida) (Figure 1G – red; examples – Figure 1—figure supplement 1C, F, I, L), to more mildly affected hearts in which the myocardial populations form a U-shaped structure, having merged at the posterior but not anterior end (Figure 1G – orange; examples Figure 1—figure supplement 1B, E, H, K). A subset of the PI3K-inhibited embryos also appear phenotypically normal (~25% for 20 μM LY, Figure 1F, G) indicating incomplete penetrance. However, increasing the concentration of PI3K inhibitor or dnPI3K mRNA increases the severity and penetrance of these phenotypes in a dose-dependent manner (Figure 1—figure supplement 1). Similarly, we confirmed that LY inhibits PI3K activity in a dose-dependent manner (Figure 1H). Thus, the severity and penetrance of cardiac fusion defects depend on the efficacy of PI3K inhibition.
Since differing modes of movement (Holtzman et al., 2007) and cellular processes such as MET (Trinh and Stainier, 2004; Jackson et al., 2017) and subduction (Ye et al., 2015) occur at distinct developmental stages during cardiac fusion, we evaluated the developmental stages over which PI3K signaling is required. Short exposures (<3 hr) just prior to 22s or starting at bud stage had no effect on cardiac fusion. However, progressively longer times of exposure ending at 22s or starting at bud stage result in correspondingly more severe phenotypes and higher penetrance (Figure 2A, B). These addition and wash-out experiments indicate that both the severity and penetrance of cardiac fusion phenotypes correlate with the duration of LY incubation and not a specific developmental stage inside the 3–20s window. Thus, the translocation of the myocardium toward the midline is responsive to both the levels and duration of PI3K signaling throughout cardiac fusion.
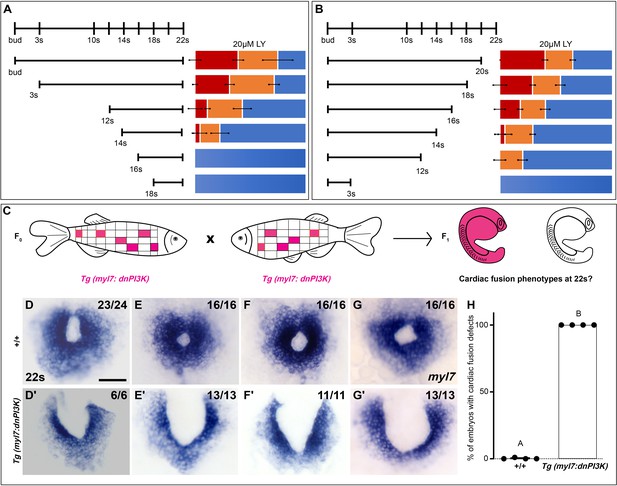
Phosphoinositide 3-kinase (PI3K) is required in the myocardium throughout cardiac fusion.
Graphical representation of the PI3K inhibitor addition (A) and wash-out (B) experiments used to determine the developmental stage over which PI3K is required. In (A) LY is added to embryos at different developmental stages and incubated until 22s, when cardiac fusion is assessed. In (B), LY is added at bud stage and washed-out at different developmental stages, after which embryos are incubated in normal media till 22s, when cardiac fusion is assessed. Bar graphs indicate the average proportion of embryos displaying different phenotypes. Blue – cardiac ring/normal; orange – fusion only at posterior end/mild phenotype, red – cardia bifida/severe phenotype. n = 45 embryos per treatment condition from three biological replicates. (C) Schematic outlines experimental design to test requirement for PI3K in the myocardium. Pink – cells with the Tg(myl7:dnPI3K) transgene. F0 animals are mosaic for the transgene, while all cells in F1 embryos either have the transgene (pink) or do not (white). The myl7 promoter restricts dnPI3K expression to the myocardium in Tg(myl7:dnPI3K) embryos. (D–G) Dorsal view of the myocardium labeled with myl7 in embryos at 22s from four different founder pairs (D–D', E–E', F–F', G–G'). F1 embryos without the Tg(myl7:dnPI3K) transgene (as determined by genotyping) display normal cardiac fusion (D–G, n = 23/24, 16/16, 16/16, 16/16, per founder pair), while F1 siblings with the Tg(myl7:dnPI3K) transgene display cardiac fusion defects (D'–G', n = 6/6, 13/13, 11/11, 13/13), indicating that PI3K signaling is required in myocardial cells. (H) Graph indicating the average % of wild-type and Tg(myl7:dnPI3K)+ embryos with cardiac fusion defects. Letter difference indicates a significant Fisher’s exact test, p = 5.56 × 10−31. Scale bar, 40 μm.
-
Figure 2—source data 1
Statistical source data for Figure 2.
- https://cdn.elifesciences.org/articles/85930/elife-85930-fig2-data1-v3.xlsx
PI3K signaling is required in the myocardium for proper cardiac fusion
Mutations affecting the specification or morphology of the anterior endoderm result in myocardial movement defects (Kupperman et al., 2000; Ye and Lin, 2013; Osborne et al., 2008; Fukui et al., 2014), revealing a non-autonomous role for the anterior endoderm in cardiac fusion. However, when PI3K signaling is inhibited with 15 or 25 μM LY starting at bud stage we did not observe differences in the expression of endoderm markers such as axial/foxa2 or Tg(sox17:egfp) compared to DMSO-treated embryos (Figure 2—figure supplement 1A–C, E–G). A detailed examination of the anterior endoderm morphology in PI3K-inhibited embryos revealed it was intact and contiguous similar to DMSO-treated embryos (Figure 2—figure supplement 1I, J) as was the average anterior endoderm width (Figure 2—figure supplement 1D, H). Thus, cardiac fusion defects in PI3K-inhibited embryos are unlikely to be due to changes in the anterior endoderm.
To determine if PI3K signaling is specifically required within the myocardium, as opposed to the endoderm, we created a myocardial-specific dominant negative transgenic construct, Tg(myl7:dnPI3K). Our experimental design is outlined in Figure 2C. In F1 embryos at 22s, derived from incrosses between Tg(myl7:dnPI3K) mosaic F0 animals, we observed embryos with normal cardiac rings and embryos with cardiac fusion defects (Figure 2D–G'). Genotyping revealed that F1 embryos with normal cardiac rings (Figure 2D–G) did not have the transgene (n = 71/71), while almost all sibling embryos with cardiac fusion defects (Figure 2D'–G') were positive for the Tg(myl7:dnPI3K) transgene (n = 43/44). And all embryos with the Tg(myl7:dnPI3K) transgene have a cardiac fusion defect (Figure 2H). (F1 embryos from four independent founder pairs were analyzed. Stable transgenics could not be propagated due to loss of viability, likely due to a requirement for PI3K signaling in cardiac contraction at later stages; Crackower et al., 2002.) Statistical analysis reveals that the Tg(myl7:dnPI3K) transgene is significantly associated with a cardiac fusion defect (Fisher’s test p = 5.56 × 10−31).
We further confirmed that PI3K signaling is active in the myocardium during cardiac fusion by using a PH domain reporter (Balla and Várnai, 2009). Fluorophores fused to PH-domains from PIP3-binding proteins translocate to the membrane when PIP3 is induced. For example, the PH-domain from BTK fused to mkate2 localizes to the region of the plasma membrane where PI3K is actively creating PIP3 (Hall et al., 2020). By expressing this reporter (myl7:PH-mkate2) mosaically in the myocardium, we found that PH-mkate2 was enriched asymmetrically at intercellular plasma membrane boundaries in DMSO-treated embryos at 20s (Figure 2—figure supplement 2A–A''', C–E, Figure 2—video 1A). However, in PI3K-inhibited embryos PH-mkate2 was localized diffusely throughout the cytoplasm or in subcellular compartments (Figure 2—figure supplement 2B–E, Figure 2—video 1B). Combined with our tissue-specific inhibition of PI3K signaling these findings suggest that PI3K signaling acts within the myocardium to regulate its movement during cardiac fusion.
PI3K signaling is responsible for the steering and velocity of myocardial movements during cardiac fusion
Our analysis points to a role for PI3K signaling in the movement of myocardial cells. To identify the properties of myocardial movement regulated by PI3K signaling, we analyzed myocardial movement by performing in vivo time-lapse imaging with the Tg(myl7:egfp) transgene, which labels myocardial cells. A time-series using hand2 expression reveals dramatic differences between DMSO- and LY-treated embryos in the translocation of the myocardium beginning after 12s (Figure 3—figure supplement 1). We thus focused our time-lapse imaging on the 14–20s developmental window. In time-lapse movies of DMSO-treated embryos, myocardial cells display coherent medially directed movement (Figure 3A–B, E, Figure 3—figure supplement 2A–A''', Figure 3—video 1) with an average velocity of 0.2334 ± 0.007 µm/min, which is consistent with previous studies (Holtzman et al., 2007; Bloomekatz et al., 2017). In PI3K-inhibited embryos myocardial cells also display coherent, coordinated movement and do move in the general direction of the midline, however they make dramatically less progress (Figure 3C, D, Figure 3—figure supplement 2B–B''', Figure 3—video 2). Quantitative analysis of these myocardial cell tracks reveals that myocardial cells are slower (0.1879 ± 0.008 µm/min) and less efficient (Figure 3E, F, Figure 3—video 3). A close analysis of the differences in speed found that they occur throughout cardiac fusion (Figure 3—figure supplement 2C) and are mostly due to defective movement along the medial–lateral axis (Figure 3—figure supplement 2E) rather than defects in angular movement occurring along the anterior–posterior axis (Figure 3—figure supplement 2F).
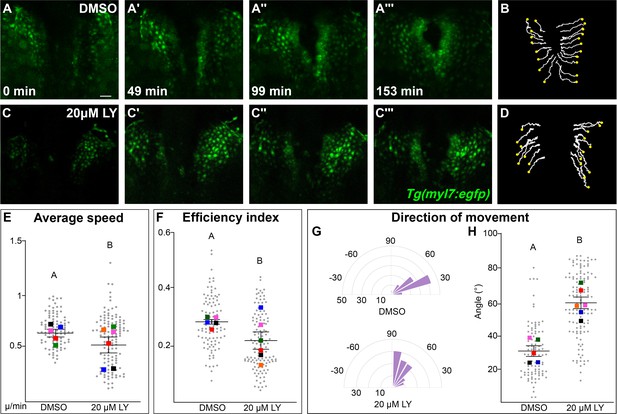
Phosphoinositide 3-kinase (PI3K) signaling regulates the medial movement and speed of the myocardium during cardiac fusion.
Time points from a representative video of myocardial cells visualized with the Tg(myl7:egfp) transgene in embryos treated with DMSO (A, B, Figure 3—video 1) or 20 μM LY (C, D, Figure 3—video 2) from bud stage to 22s. 3D reconstructions of confocal slices (A, C) reveal changes in conformation and location of the myocardium at three major stages of cardiac fusion: early medial movement toward the embryonic midline (A–A', C–C'), posterior merging of bilateral populations (A'', C'') and anterior merging to form a ring (A''', C'''). Representative tracks (B, D) show the paths of a subset of myocardial cells over ~2.5 hr. Yellow dots indicate the starting point of each track. Graphs depict average speed (E), efficiency index (F), and angle of movement (G, H) of myocardial cells. Angular movement along the anterior–posterior axis does not distinguish anterior from posterior movement (G, H). Myocardial cells in PI3K-inhibited embryos show an overall direction of movement that is angular (60–90°) and is slower than in DMSO-treated embryos. 96 and 125 cells were analyzed from five DMSO- and six 20 μM LY-treated embryos, respectively. Gray dots – individual cells; color squares – average per embryo. Average of embryos and standard error (shown in E, F, H). Two-sample t-test, letter change indicates p < 0.05. Scale bars, 60 μm. Quantification details in the methods. Raw data and full p-values included in the source file.
-
Figure 3—source data 1
Statistical source data for quantification of myocardial movement behaviors in Figure 3E–H.
- https://cdn.elifesciences.org/articles/85930/elife-85930-fig3-data1-v3.xlsx
The most dramatic difference between PI3K-inhibited and DMSO-treated myocardial cells is in the direction of their movement. Tracks of myocardial cells in DMSO-treated embryos are predominately oriented in a medial direction (average of 31.1 ± 1.65°), while tracks in LY-treated embryos are mostly oriented in an angular anterior direction (60.6 ± 1.73°, p-value = 2.77 × 10−12, Figure 3G, H). These differences occur mainly in the early stages of cardiac fusion when wild-type myocardial movement is mostly medial (Figure 3—figure supplement 2D). Altogether, this analysis of myocardial cell tracks suggests that PI3K signaling is responsible for both steering and propelling myocardial cells toward the midline.
Myocardial membrane protrusions are medially polarized by PI3K signaling
The role of PI3K signaling in regulating the polarity of migratory protrusions in the dorsal epithelium of Drosophila embryos and in the prechordal plate of zebrafish embryos (Montero et al., 2003; Garlena et al., 2015) along with previous reports of the existence of myocardial membrane protrusions during heart tube formation (Dominguez et al., 2023; Ye et al., 2015) led us to look for these protrusions during cardiac fusion and to examine if they are disrupted in PI3K-inhibited embryos. To visualize membrane protrusions in the myocardium, we performed in vivo time-lapse imaging during cardiac fusion of embryos injected with myl7:lck-egfp plasmid DNA in order to mosaically label the plasma membrane of myocardial cells. Despite myocardial cells being connected via intercellular junctions (Trinh and Stainier, 2004; Bloomekatz et al., 2017), we observed that the lateral edges of myocardial cells in wild-type/DMSO-treated embryos are highly dynamic; transitioning from appearing smooth and coherent to undulating and extending finger-like membrane protrusions away from the cell (Figure 4A–A'''', Figure 4—video 1). These protrusions actively extend and retract, and are prevalent occurring on average 20.3 ± 6.7 times per hour per cell and lasting for an average of 2.3 ± 0.6 min (Figure 4A). We observed different types of protrusion morphologies, including thin finger-like protrusions and wide protrusions which have a wider base (Figure 4—figure supplement 1A). In LY-treated embryos we observed similar types of membrane protrusions extending from myocardial cells (Figure 4B–B'''', Figure 4—figure supplement 1B, Figure 4—video 1). These protrusions occur at a similar rate to those found in DMSO-treated embryos (17 ± 7.4 per hour per cell, p-value = 0.36), but with slightly longer persistence (3.23 ± 0.84 min, p-value = 0.008).
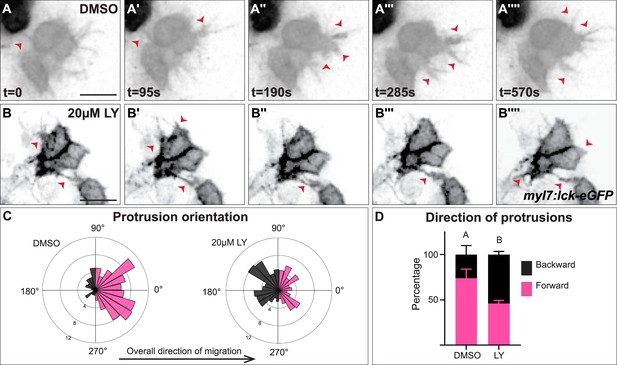
Myocardial membrane protrusions are misdirected in phosphoinositide 3-kinase (PI3K)-inhibited embryos.
(A–B'''') timepoints from representative videos (see Figure 4—video 1) of myocardial cells whose membrane has been labeled with myl7:lck-eGFP (black), medial to the right, in a DMSO- (A–A'''') or a 20 μM LY- (B–B'''') treated embryo. Red arrowheads indicate representative protrusions, which are mostly oriented medially, coincident with the direction of movement in DMSO-treated embryos (A–A'''') but are oriented in all directions in LY-treated embryos (B–B''''). Rose (C) and bar (D) graphs displaying the orientation of membrane protrusions in DMSO- (left) or LY- (right) treated embryos. The length of each radial bar in (C) represents the percentage of protrusions in each bin. Bar graph displays the total percentage of forward or backward protrusions. Forward protrusions: 270–90°, pink. Backward protrusions: 90–270°, black. n = 425 protrusions from 11 cells in 5 embryos (DMSO), and 480 protrusions from 11 cells in 4 embryos (20 μM LY). Fisher’s exact test, p-value 1.8 × 10−5. Mean ± standard error. Scale bar, 30 μm. Raw data included in the source file.
-
Figure 4—source data 1
Statistical source data for quantification of myocardial protrusion properties.
- https://cdn.elifesciences.org/articles/85930/elife-85930-fig4-data1-v3.xlsx
We further observed that both types of membrane protrusions in DMSO-treated embryos occur predominantly in the medial direction (77.25 ± 21.76% of protrusions were in the forward/medial direction, Figure 4A–A'''', C, D, and Figure 4—figure supplement 1C, D), suggesting an association with the medial movement of the myocardial tissue. In contrast, in LY-treated embryos myocardial membrane protrusions do not display the same medial polarity, instead extending from all sides of a myocardial cell equally (only 46 ± 11.6% of protrusion were in the forward direction, Figure 4B–B'''', C, D and Figure 4—figure supplement 1C, D). The finding that myocardial membrane protrusions are medially polarized in wild-type embryos but not in PI3K-inhibited embryos where myocardial cells are misdirected and slower to reach the midline suggests that PI3K signaling helps to steer and propel myocardial cells toward the midline through the polarization of these active protrusions.
PI3K signaling is regulated by Pdgfra during cardiac fusion
We next investigated whether Pdgfra activates PI3K signaling to regulate myocardial movement. We found that PI3K activity as measured by the ratio of phospho-AKT to AKT levels (Alessi et al., 1996) is severely diminished in pdgfra mutant embryos during cardiac fusion (Figure 5A). Conversely, when Pdgfra activity is increased during cardiac fusion through the over-expression of pdgfaa, PI3K activity is upregulated (Figure 5B).
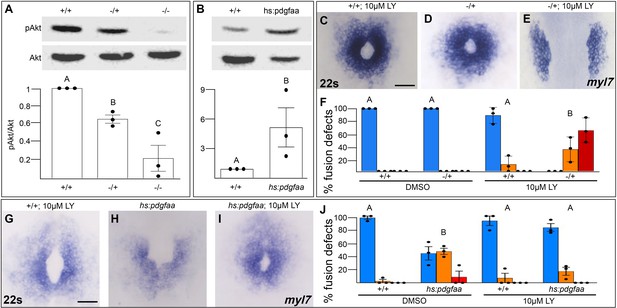
Pdgfra activates and genetically interacts with phosphoinositide 3-kinase (PI3K) signaling to regulate cardiac fusion.
(A, B) Immunoblot and ratiometric analysis of phosphorylated Akt (pAkt) compared to total Akt levels reveals reduced pAkt levels in loss-of-function pdgfrask16 heterozygous (−/+) or homozygous (−/−) mutant embryos at 22s (A), and elevated pAkt levels at 22s when PDGF signaling is activated with the Tg(hs:pdgfaa) transgene (B). Bar graphs display averages from three separate experiments. (C–E, G–I) Dorsal views, anterior to the top, of the myocardium labeled with myl7 at 22s. In contrast to a normal ring of myocardial cells found in wild-type embryos treated with 10 μM LY starting at bud stage (C) or in pdgfra heterozygous embryos (D), when pdgfra heterozygous mutants are exposed to 10 μM LY, cardiac fusion is defective with embryos displaying severe phenotypes such as cardia bifida (E). Furthermore, the percent of cardiac fusion defects observed in Tg(hs:pdgfaa) embryos heat-shocked at bud stage (H) is greatly decreased when heat-shocked Tg(hs:pdgfaa) embryos are exposed to 10 μM LY at bud stage (I). (F, J) Bar graphs depict the average distribution of cardiac fusion defects from the indicated genotypes. The total number of embryos examined over three separate replicates are 47 (DMSO, +/+), 25 (DMSO, −/+), 36 (10 μM LY, +/+), 31 (10 μM LY, −/+), 49 (heat-shock, DMSO, +/+), 57 (heat-shock, DMSO, Tg(hs:pdgfaa)), 51 (heat-shock, 10 μM LY, +/+), 57 (heat-shock, 10 μM LY, Tg(hs:pdgfaa)). Blue – cardiac ring/normal; orange – fusion only at posterior end/mild, red – cardia bifida/severe. Bar graphs, mean ± standard error. One-way analysis of variance (ANOVA) (A, F, J) or Student’s t-test (B), letter change indicates p < 0.05. Scale bar, 60 μm. Raw data and full p-values included in the source file.
-
Figure 5—source data 1
Statistical source data for Figure 5A, B, F, J.
- https://cdn.elifesciences.org/articles/85930/elife-85930-fig5-data1-v3.xlsx
-
Figure 5—source data 2
Original immunoblots used in Figure 5A, B (raw, uncropped) with and without labeling.
- https://cdn.elifesciences.org/articles/85930/elife-85930-fig5-data2-v3.zip
To determine if Pdgfra’s influence on PI3K activity is important for myocardial movement toward the midline, we tested whether they functionally interact to regulate cardiac fusion. When pdgfra heterozygous mutant embryos are exposed to DMSO cardiac fusion occurs normally (Figure 5D, F), even though there is a small reduction in PI3K activity (Figure 5A). Similarly, in wild-type embryos exposed to 10 μM LY, PI3K activity is modestly reduced (Figure 1H) and a small percent of embryos display mild cardiac fusion defects (average of 10.9 ± 7.39% of 10 μM LY-treated embryos display mild U-shaped cardiac fusion defects, n = 36, 3 replicates, Figure 5C, F). However, when pdgfra heterozygous mutant embryos are exposed to 10 μM LY, there is a synergistic increase in both the severity and penetrance of cardiac fusion defects. 100% of pdgfra heterozygous embryos exposed to 10 μM LY display cardiac fusion defects, with the majority of embryos displaying severe cardia bifida phenotypes (Figure 5E, F). Furthermore, we found that cardiac fusion defects caused by exogenous expression of pdgfaa (Figure 5H, J, Bloomekatz et al., 2017), which causes increased PI3K activity (Figure 5B), could be rescued by a sub-phenotypic reduction in PI3K activity (Figure 5I, J). Together these results suggest that PDGF signaling activates PI3K activity to promote myocardial movement toward the midline.
Discussion
Our studies reveal an intrinsic PI3K-dependent mechanism by which the myocardium moves toward the midline during the formation of the primitive heart tube. Together with our previous studies revealing a role for the PDGF pathway in facilitating communication between the endoderm and myocardium (Bloomekatz et al., 2017), our current work suggests a model in which Pdgfra in the myocardium senses signals (PDGF ligands) from the endoderm and via the PI3K pathway directs myocardial movement toward the midline through the production of medially oriented membrane protrusions. While genetic and imaging studies in zebrafish and mice (Trinh and Stainier, 2004; Kupperman et al., 2000; Molkentin et al., 1997; Ye and Lin, 2013; Cui et al., 2009; Ye et al., 2015; Aleksandrova et al., 2015; Garavito-Aguilar et al., 2010; Arrington and Yost, 2009) along with embryological studies in chicks and rats (Goss, 1935; Rosenquist, 1970; Varner and Taber, 2012; Moreno-Rodriguez et al., 2006) have identified the importance of extrinsic influences – such as the endoderm and extracellular matrix, on myocardial movement to the midline, our studies using tissue-specific techniques identifies an active role for myocardial cells, providing insight into the balance of intrinsic and extrinsic influences that regulate the collective movement of the myocardial tissue during heart tube formation.
Specifically, we found a requirement for PI3K signaling in cardiac fusion. By examining both loss-of-PI3K activity (Figure 1) and gain-of-PI3K activity through loss-of-pten (Figure 1—figure supplements 5 and 6) our studies indicate that appropriate PIP3 levels are required for the proper movement of cardiomyocytes to the midline. This analysis is complemented by previous studies in mice examining Pten mutants (Bloomekatz et al., 2012). Our spatial and temporal experiments further reveal a requirement for PI3K activity specifically in the myocardium and throughout the duration of cardiac fusion (Figure 2). Furthermore, the lack of phenotype caused by inhibition of mTOR (Figure 1—figure supplement 3) suggests that mTOR and its downstream signals are unlikely to be important PI3K effectors in regulating cardiomyocyte movement.
When we examined the cellular behaviors affected by loss of PI3K signaling we observed a mild disorganization of myocardial intercellular junctions (Figure 1—figure supplement 4A–F). This finding is consistent with previous studies linking epithelial polarity to PI3K signaling (Krahn, 2020). However, myocardial cells defective in apical–basal polarity still form a cardiac ring (Horne-Badovinac et al., 2001; Rohr et al., 2006), suggesting that an apical–basal defect is unlikely to be the primary reason for myocardial movement defects. Instead, our studies showing that PI3K-inhibited myocardial cells move slower and are misdirected during the early stages of cardiac fusion indicate a role for PI3K signaling in the steering of myocardial movements medially toward the midline. Specifically, we found that DMSO-treated myocardial cells in our study display an average direction of 31.1 ± 1.65° compared to PI3K-inhibited embryos which display an average of 60.6 ± 1.73°. Our wild-type tracks results are grossly similar to previous published studies (Holtzman et al., 2007; Bloomekatz et al., 2017). Differences in the extent of the medial orientation between these studies are likely due to experimental variations in normalization used to account for drift as well as small differences in the developmental stages over which cells were tracked. Our finding that steering in PI3K-inhibited embryos is perturbed in the early stages of cardiac fusion is consistent with the previous identification of different types of myocardial movement during different stages of cardiac fusion (Holtzman et al., 2007) and suggests that PI3K signaling could be part of a distinct molecular mechanism that drives these early medial phases of myocardial movement. Loss-of-function pdgfra mutants, like PI3K-inhibited embryos, also display defects in the directional movement of myocardial cells (Bloomekatz et al., 2017), although the phenotype in pdgfra mutants can be more severe. These differences in severity could be a result of differences in the extent of loss-of-function and/or differences in the ability of other genes to compensate. Indeed, our interaction studies (Figure 5) suggest that Pdgfra and PI3K signaling work together to regulate cardiac fusion.
Myocardial membrane protrusions during cardiac fusion were postulated by De Haan et al. in 1967 as a mechanism by which myocardial cells move toward the midline (DeHaan, 1967). Using mosaic membrane labeling of myocardial cells to visualize membrane protrusions, we have observed myocardial membrane protrusions that are oriented in the medial direction in a PI3K-dependent manner, confirming De Haan’s hypothesis. These studies are complemented by previous studies in zebrafish which observed myocardial protrusions prior to and after cardiac fusion (Ye et al., 2015; Rohr et al., 2008) as well as recent studies in the mice (Dominguez et al., 2023) indicating that these cellular processes are likely conserved. Myocardial membrane protrusions have been discovered during trabeculation (Staudt et al., 2014), cardiac regeneration (Morikawa et al., 2015; Aharonov et al., 2020) and during the muscularization of the outflow tract (van den Hoff et al., 1999). Indeed, similar observations of PI3K signaling orienting and stimulating protrusion formation in migrating Dictyostelium and neutrophil cells as well as in the collective movement of endothelial tip cells, the prechordal plate, and the dorsal epithelium (Iijima and Devreotes, 2002; Yoo et al., 2010; Montero et al., 2003; Garlena et al., 2015; Dumortier et al., 2012; Graupera et al., 2008) support a conserved role for PI3K signaling in regulating protrusion formation.
However, the question of how active membrane protrusions facilitate the collective medial movement of the myocardium to the midline remains to be addressed. Our studies indicate that directionality and to a lesser extent velocity and efficiency are compromised, when membrane protrusions are improperly oriented in PI3K-inhibited embryos (Figure 3). These observations could suggest that the observed membrane protrusions are force generating, similar to protrusions from leader cells in the lateral line or in endothelial and tracheal tip cells (Caussinus et al., 2008; Dalle Nogare et al., 2020; Qin et al., 2021). Alternatively, these protrusions could act more like filopodia sensing extrinsic signals and the extracellular environment (Heckman and Plummer, 2013). Future studies examining myocardial protrusions and their role in the biomechanical dynamics of the myocardium will help to elucidate the role of membrane protrusions in the collective movement of the myocardium during cardiac fusion.
Our studies utilize small pharmacological molecules to investigate the role of PI3K signaling in cardiac fusion (Figure 1). These inhibitors have been widely used and have several advantages including the ability to target multiple class I PI3K complexes which are known to compensate for each other (Juss et al., 2012), and the ability to avoid earlier pleiotropic effects by temporally restricting their usage (Montero et al., 2003; Leslie et al., 2007). Nevertheless, small molecule inhibitors can also affect non-targeted proteins resulting in off-target phenotypic artifacts. We have tried to mitigate these limitations by utilizing multiple different PI3K inhibitors (Figure 1B–D), ensuring a dose–response (Figure 1—figure supplement 1A–I, N, O), directly testing the role of known off-targets (Figure 1—figure supplement 3) and utilizing a dominant-negative construct (Figure 1E, Figure 1—figure supplement 1J–L, P). However, these complementary experiments have their own limitations and thus we are unable to completely rule-out the role of a combinatorial inhibitory effect on cardiac fusion. We look forward to the creation of a genetic model that will help verify our findings.
Overall, our studies delineate a role for the PDGF–PI3K pathway in the mechanisms by which myocardial precursors sense and respond to extracellular signals to move into a position to form the heart. These mechanisms are likely relevant to other organ progenitors including endothelial precursors, endodermal progenitors, and neuromasts; all of which must move from their location of specification to a different location for organ formation. Although varying in their morphogenesis, many of these movements are collective in nature. Indeed, a similar Pdgfra–PI3K signaling cassette is important in the collective directional migration of several organ progenitors including the migration of mesoderm and neural crest cells (Montero et al., 2003; Yang et al., 2008; McCarthy et al., 2013; Bahm et al., 2017; Symes and Mercola, 1996; Klinghoffer et al., 2002; He and Soriano, 2013; Nagel and Winklbauer, 2018). RTK–PI3K pathways are also important across several cardiac developmental processes, including epicardial development, cardiac neural crest addition, cardiomyocyte growth, cardiac fibroblast movement, and cardiomyocyte contraction (Crackower et al., 2002; Kim et al., 2010; Sato et al., 2011; Ivey et al., 2019; McMullen et al., 2003; Shioi et al., 2000). Similarly, PDGF–PI3K and more generally RTK–PI3K signaling cassettes are activated in several diseases including glioblastomas, gastrointestinal stromal tumors, and cardiac fibrosis (Wang et al., 1997; Cheng et al., 2009; Fan et al., 2014; Lennartsson et al., 2005). Thus, the role of this RTK–PI3K cassette in sensing and responding to extracellular signals is likely to be broadly relevant to the etiology of a wide array of developmental processes as well as congenital diseases.
Materials and methods
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Danio rerio) | Tg(myl7: eGFP)twu34 | Huang et al., 2003 | twu34; RRID: ZFIN_ZDB-GENO-050809-10 | Transgenic |
| Genetic reagent (Danio rerio) | Tg(sox17:eGFP)ha01 | Mizoguchi et al., 2008 | ha01; RRID: ZFIN_ZDB-GENO-080714-2 | Transgenic |
| Genetic reagent (Danio rerio) | Tg(hsp70l:pdgfaa-2A-mCherry;cryaa:CFP)sd44 | Bloomekatz et al., 2017 | sd44; ZDB-ALT-170425-5 | Transgenic |
| Genetic reagent (Danio rerio) | ref (pdgfrask16) | Bloomekatz et al., 2017 | sk16; ZDB-ALT-170329-1 | Mutant |
| Genetic reagent (Danio rerio) | ptenahu1864 | Faucherre et al., 2008 | hu1864; ZDB-ALT-080910-1 | Mutant |
| Genetic reagent (Danio rerio) | ptenbhu1435 | Faucherre et al., 2008 | hu1435; ZDB-ALT-080910-2 | Mutant |
| Genetic reagent (Danio rerio) | Tg(myl7:dnPI3K; Cryaa:CFP) | This paper | Transgenic, see Materials and methods | |
| Genetic reagent (Danio rerio) | Tg(myl7:lck-emgfp) | This paper | Transgenic, see Materials and methods | |
| Recombinant DNA reagent | pBSRN3-∆p85 | Carballada et al., 2001 | ||
| Other | myl7 | Yelon et al., 1999 | ZDB-GENE-991019-3 | mRNA probe |
| Other | axial | Strähle et al., 1993 | ZDB-GENE-980526-404 | mRNA probe |
| Other | hand2 | Yelon et al., 2000 | ZDB-GENE-000511-1 | mRNA probe |
| Chemical compound, drug | LY294002 | Millipore-Sigma | Cat# 154447-36-6 | |
| Chemical compound, drug | Dactolisib | Millipore-Sigma | Cat# 915019-65-7 | |
| Chemical compound, drug | Pictilisib | Millipore-Sigma | Cat# 957054-30-7 | |
| Chemical compound, drug | Rapamycin | Selleckchem | Cat# S1039 | |
| Chemical compound, drug | VO-Ohpic trihydrate | Selleckchem | Cat# S8174 | |
| Antibody | phospho-AKT (Rabbit monoclonal) | Cell Signaling | Cat# 4060, RRID: AB_2315049 | WB(1:2000) |
| Antibody | pan-AKT (Rabbit monoclonal) | Cell Signaling | Cat# 4691, RRID: AB_915783 | WB (1:2000) |
| Antibody | Anti-rabbit IgG HRP-conjugated (Goat polyclonal) | Cell Signaling | Cat# 7074, RRID: AB_2099233 | WB (1:5000) |
| Antibody | anti-GFP (Chicken polyclonal) | Abcam | Cat# ab13970, RRID: AB_300798 | IF (1:1000) |
| Antibody | anti-ZO-1 (Mouse monoclonal) | Thermo Fisher Scientific | Cat# 33–9100, RRID: AB_87181 | IF (1:200) |
| Antibody | anti-chicken-488 (Goat polyclonal) | Thermo Fisher Scientific | Cat# A32931TR, RRID: AB_2866499 | IF (1:300) |
| Antibody | anti-mouse-647 (Goat polyclonal) | Thermo Fisher Scientific | Cat# A32728, RRID: AB_2633277 | IF (1:300) |
| Commercial assay or kit | Cell Death detection kit, TMR red | Millipore Sigma | Cat# 12156792910 | |
| Commercial assay or kit | Click-&-Go Cell Proliferation Assay Kit | Click Chemistry Tools | Cat# 1328 | |
| Software, algorithm | mTrackJ | Meijering et al., 2012 | ImageJ Plugin for motion tracking and analysis | |
| Software, algorithm | Correct 3D Drift | Parslow et al., 2014 | ImageJ Plugin for sample drift correction | |
| Software, algorithm | Prism 10.0.2 | GraphPad | RRID: SCR_002798 | Data visualization and statistics software |
| Software, algorithm | Leica | LASX | Leica Application Suite X, RRID: SCR_013673 | Microscope image processing software |
Material availability
Request a detailed protocolMaterials not available commercially are available upon request to Dr. Joshua Bloomekatz.
Zebrafish husbandry, microinjections, and plasmid construction
Request a detailed protocolAll zebrafish work followed protocols approved by the University of Mississippi IACUC (protocol #21-007). Wildtype embryos were obtained from a mixed zebrafish (Danio rerio) AB/TL background. The following transgenic and mutant lines of zebrafish were used: Tg(myl7: eGFP)twu34 (RRID: ZFIN_ZDB-GENO-050809-10) (Huang et al., 2003), Tg(sox17:eGFP)ha01 (ZFIN_ZDB-GENO-080714-2) (Mizoguchi et al., 2008), Tg(hsp70l:pdgfaa-2A-mCherry;cryaa:CFP)sd44 abbreviated hs:pdgfaa (ZDB-ALT-170425-5), ref (pdgfrask16) (ZDB-ALT-170329-1) (Bloomekatz et al., 2017), ptenahu1864 (ZDB-ALT-080910-1), and ptenbhu1435 (ZDB-ALT-080910-2) (Faucherre et al., 2008). All embryos were incubated at 28.5°C unless otherwise noted. Transgenic Tg(myl7:dnPI3K; Cryaa:CFP) or Tg(myl7:lck-emgfp) F0 founders were established using standard Tol2-mediated transgenesis (Fisher et al., 2006). F0 founder pairs were screened by intercrosses looking for a high percentage of F1 embryos with CFP+ eyes and cardiac edema or emGFP+ hearts, respectively. For Tg(myl7:dnPI3K; Cryaa:CFP), stable transgenic lines could not be propagated due to loss of viability. Based on the germline mosaicism of the F0 parents, only a proportion of the F1 embryos are expected to have the transgene. Embryos from four different F0 pairs were analyzed for cardiac fusion phenotypes. Due to germ-line mosaicism, F1 embryos were genotyped after in situ hybridization for the presence of the transgene using standard PCR genotyping. Primer sequences are provided in Table 1. ref (pdgfrask16), ptenahu1864, and ptenbhu1435 were genotyped as outlined in Bloomekatz et al., 2017; Jung et al., 2021, respectively.
Primers for genotyping and cloning.
| Name | Sequence (5′–3′) | |
|---|---|---|
| Primers to screen for the Tg(myl7:dnPI3K) transgene in F1 embryos | dnPI3K_F1 | gcgggaagaggacattgact |
| dnPI3K_R1 | gcgggaagaggacattgact | |
| Primers to clone lck-emGFP into the middle-entry vector of the tol2 gateway system | Hi_lck_1F | cagtcgactggatccggtacagatccgctagccaccatg |
| Hi_lck_1R | cagtcgactggatccggtacagatccgctagccaccatg | |
| Hi_emgfp_2F | ggtcgccaccgtgtccaagggcgaggag | |
| Hi_emgfp_2R | ggtcgccaccgtgtccaagggcgaggag | |
| Primers to replace actc1b promoter with myl7 promoter in Addgene plasmid 109501 | Hi_CbPHmkate2_F | ggctgaaaagcaatcctgcagtgaccaaagcttaaatcagttg |
| Hi_CbPHmkate2_R | ctctccagaatcactgcggccatggccatggtggctacggatc |
Truncated p85 (dnPI3K) capped mRNA was synthesized from the pBSRN3-∆p85 construct (Carballada et al., 2001) and injected at the one-cell stage. To mosaically label cells in the myocardium for protrusion imaging or PI3K-reporter activity, myl7:lck-eGFP (30 ng/μl) or myl7:PH-mkate2 (50 ng/μl) plasmid DNA was injected along with Tol2 transposase (40 ng/μl) into Tg(myl7:eGFP) or Tg(myl7:lck-emgfp) heterozygous embryos, respectively, at the one-cell stage and embryos were subsequently allowed to develop at 28.5°C.
Plasmids were constructed by using Hifi assembly (NEB, E2621) to transfer lck-eGFP (Chertkova et al., 2017) or a truncated version of p85 (Carballada et al., 2001) into the middle-entry vector of the tol2 gateway system (Kwan et al., 2007), which were verified by sequencing. Primer sequences are provided in Table 1. Then gateway recombination between p5E-myl7 promoter, the constructed middle-entry clones, p3E-polyA and either pDESTTol2pA2 (Kwan et al., 2007) or pDESTTol2pA4-Cryaa:CFP (Bloomekatz et al., 2017) was used to produce the plasmids myl7:lck-eGFP or myl7:dnPI3K; Cryaa:CFP, respectively. The myl7:PH-mkate2 plasmid was created by using Hifi assembly to replace the actc1 promoter with the myl7 promoter in the addgene plasmid #109501 (Hall et al., 2020).
Inhibitor treatments
Request a detailed protocolThe following inhibitors were used: LY294002 (LY, Millipore-Sigma 154447-36-6), Dactolisib (Dac, Millipore-Sigma 915019-65-7), Pictilisib (Pic, Millipore-Sigma 957054-30-7), Rapamycin (Rap, Selleckchem S1039), and VO-Ohpic trihydrate (VOOH, Selleckchem S8174). For each treatment, inhibitors were freshly diluted serially from stocks such that the same percentage (0.1%) of DMSO was added to 1× E3 in glass vials. 0.1% DMSO was used as a control. 15 dechorionated embryos per vial were incubated in the dark at 28.5°C. In the course of these studies, we noticed that incubation with pharmacological PI3K inhibitors caused a delay in trunk elongation and somite formation along with defects in cardiac fusion (Figure 1—figure supplement 2). To ensure our analysis was not obfuscated by a developmental delay, we used somite number to stage match embryos. PI3K-inhibited embryos thus develop approximately 2–3 hr longer than DMSO-treated embryos, prior to analysis.
Immunoblot, immunofluorescence, in situ hybridization
Request a detailed protocolEmbryos at 22s were prepared for immunoblots by deyolking (Purushothaman et al., 2019). Primary and secondary antibodies include anti-phospho-AKT (1:2000, Cell Signaling 4060, RRID: AB_2315049), anti-pan-AKT (1:2000, Cell Signaling 4691, RRID: AB_915783), and anti-rabbit HRP conjugated (1:5000, Cell Signaling 7074, RRID: AB_2099233). To identify pdgfra/ref heterozygous and homozygous embryos, embryo trunks were clipped and genotyped as described (Bloomekatz et al., 2017). The body of the embryo including the heart was snapped frozen and stored at −80°C. After genotyping, embryos were pooled via their genotype and analyzed via immunoblot. To activate Pdgfra, embryos expressing the Tg(hsp70l: pdgfaa-2A-mCherry) transgene were heat-shocked at bud stage as described (Bloomekatz et al., 2017) and collected at 22s. pAKT and AKT immunoblots were visualized (Azure 600 Imaging system, Azure Biosystems) and quantified using ImageJ (Stael et al., 2022). pAkt to Akt ratios were normalized to DMSO.
Immunofluorescence analysis was performed on transverse sections using standard cryoprotection, embedding, and sectioning (Garavito-Aguilar et al., 2010). Primary, secondary antibodies and dyes include: anti-GFP (1:1000, Abcam ab13970, RRID: AB_300798), anti-ZO-1 (1:200, Thermo Fisher Scientific 33-9100, RRID: AB_87181), donkey anti-chicken-488 (1:300, Thermo Fisher Scientific A32931TR, RRID: AB_2866499), and donkey anti-mouse-647 (1:300, Thermo Fisher Scientific A32728, RRID:AB_2633277). TUNEL was performed using the TMR red Cell Death detection kit (Millipore Sigma 12156792910). Addition of DNaseI was used to confirm we could detect apoptotic cells. EdU staining was adapted from Kimmel and Meyer, 2010; Schindler et al., 2014 using the Click-&-Go Cell Proliferation Assay Kit (Click Chemistry Tools 1328), with the following modifications: dechorionated 16 hpf embryos were incubated in 0.5 mM EdU for 1 hr at 4°C, rinsed in E3 media multiple times, and then incubated at 28.5 C till 22s.
In situ hybridization was performed using standard protocols (Alexander et al., 1999), with the following probes: myl7 (ZDB-GENE-991019-3), axial (ZDB-GENE-980526-404), and hand2 (ZDB-GENE-000511-1). Images were captured with Zeiss Axio Zoom V16 microscope (Zeiss) and processed with ImageJ.
Fluorescence imaging
Request a detailed protocolTo analyze cardiac fusion (Figure 1A'–E'), Tg(myl7:eGFP) embryos were fixed, manually deyolked and imaged with a Leica SP8 X microscope. To analyze the anterior endoderm (Figure 2—figure supplement 1E–G), Tg(sox17: eGFP) embryos were fixed and imaged with an Axio Zoom V16 microscope (Zeiss).
For live imaging, Tg(myl7:eGFP) embryos were exposed to DMSO or 20 μM LY at bud stage and mounted at 12 somite stage as described (McCann et al., 2022). Mounted embryos were covered with 0.1% DMSO/20 μM LY in Tricaine-E3 solution and imaged using a Leica SP8 X microscope with a HC PL APO ×20/0.75 CS2 objective in a chamber heated to 28.5°C. GFP and brightfield stacks were collected approximately every 4 min for 3 hr. After imaging, embryos were removed from the mold and incubated for 24 hr in E3 media at 28.5°C. Only embryos that appeared healthy 24 hrs post imaging were used for analysis. The tip of the notochord was used as a reference point to correct embryo drift in the Correct 3D direct ImageJ plugin (Parslow et al., 2014). Embryos were handled similarly for imaging protrusions and PH-mkate2 localization, except 15 confocal slices of 1 μm thickness were collected every 1.5 min or 3.5 min, respectively, with a HC PL APO ×40/1.10 CS2 objective.
Image analysis
Request a detailed protocolTo analyze developmental delay, embryonic length (Figure 1—figure supplement 2) was measured from the anterior tip of the head to the posterior tip of the tail of each embryo using the free-hand tool of ImageJ. To analyze the anterior endoderm, endoderm width was measured at a point that was 300 µm anterior to where the two sides of the endoderm intersect. The distance between the hand2 expressing domains was measured at three equidistant positions (~200 µm apart) along the anterior–posterior axis. Tg(myl7:eGFP)+ cardiomyocytes were counted from blinded and non-blinded 3D confocal images of 20s embryos from four biological replicates using the cell counter addon in ImageJ. No difference between the blinded and non-blinded replicates was detected. Cell proliferation index was calculated as Tg(myl7:eGFP)+; EdU+ cells divided by the total number of Tg(myl7:eGFP)+ cells.
For live imaging of cell movements – the mTrackJ addon in ImageJ (Meijering et al., 2012) was used. 20–25 cells per embryo whose position could be determined at each timepoint were chosen from the two most medial columns of myocardial cells on each side of the embryos. From these tracks, cell movement properties including overall displacement, velocity (displacement/time), speed (distance/time), efficiency (displacement/distance), and direction (atan(abs(Δy)/Δx) × 57.295) – medial–lateral and angular, were calculated. Rose plots and graphs in Figure 3G, H consider angular movement, along the anterior–posterior axis, irrespective of its direction. In these plots, individual cells are grouped into 6 bins based on their net direction of movement; the length of each radial bar represents the percentage of cells in each bin.
To quantitate the fluorescent intensity of lck-eGFP and PH-mkate2 – the straight-line function in ImageJ was used to draw a line extending perpendicular from the middle of the most intense region of mkate2 enrichment to the end of the cell. The fluorescence intensity along this line for both eGFP and mkate2 was measured using the plot profile addon.
For live imaging of myocardial membrane protrusions – stacks were processed in Leica LAS X and/or Imaris Viewer (Bitplane) to position the medial edge to the right of the image. Videos of the myocardium were inspected frame by frame in ImageJ for a protrusion. Only cells that were not neighbored by other labeled cells on their medial and lateral edges were analyzed. Each protrusion was classified as either thin (longer than wide) or wide (wider than long). The direction of a protrusion was measured using the ‘straight line’ function to draw a line from the center of the bottom of the protrusion to the tip. All protrusions of each cell over the entire recording were measured. Graphs, cartoons, and figures were created with Prism (GraphPad), Excel (Microsoft), and Indesign (Adobe).
Statistics and replication
Request a detailed protocolAll statistical analysis was performed in R or Prism (GraphPad). In R the following functions were used T.test(), TukeyHSD(), and fisher.test(). Sample sizes were determined based on prior experience with relevant phenotypes and standards within the zebrafish community. Deviation from the mean is represented as standard error mean or box-whisker plots. In box-whisker plots, the lower and upper ends of the box denote the 25th and 75th percentile, respectively, with a horizontal line denoting the median value and the whiskers indicating the data range. All results were obtained from at least three separate biological replicates, blinded and non-blinded. All replicates are biological. Samples were analyzed before biological sex is determined (Wang et al., 2007). Raw data and full p-values included in the source files.
Data availability
All data are included in the manuscript and supporting files. Source data files have been provided for all figures.
References
-
ERBB2 drives YAP activation and EMT-like processes during cardiac regenerationNature Cell Biology 22:1346–1356.https://doi.org/10.1038/s41556-020-00588-4
-
The endoderm and myocardium join forces to drive early heart tube assemblyDevelopmental Biology 404:40–54.https://doi.org/10.1016/j.ydbio.2015.04.016
-
Mechanism of activation of protein kinase B by insulin and IGF-1The EMBO Journal 15:6541–6551.https://doi.org/10.1002/j.1460-2075.1996.tb01045.x
-
casanova plays an early and essential role in endoderm formation in zebrafishDevelopmental Biology 215:343–357.https://doi.org/10.1006/dbio.1999.9441
-
Visualization of cellular phosphoinositide pools with GFP-fused protein-domainsCurrent Protocols in Cell Biology Chapter 24:Unit 24.4.https://doi.org/10.1002/0471143030.cb2404s42
-
Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002The EMBO Journal 15:5256–5267.
-
Loss of Pten promotes angiogenesis and enhanced vegfaa expression in zebrafishDisease Models & Mechanisms 6:1159–1166.https://doi.org/10.1242/dmm.012377
-
Dynamic positional fate map of the primary heart-forming regionDevelopmental Biology 332:212–222.https://doi.org/10.1016/j.ydbio.2009.05.570
-
Myocardial lineage developmentCirculation Research 107:1428–1444.https://doi.org/10.1161/CIRCRESAHA.110.227405
-
Role of PDGFR-β/PI3K/AKT signaling pathway in PDGF-BB induced myocardial fibrosis in ratsAmerican Journal of Translational Research 6:714–723.
-
The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancerJournal of Medicinal Chemistry 51:5522–5532.https://doi.org/10.1021/jm800295d
-
Exploring the specificity of the PI3K family inhibitor LY294002The Biochemical Journal 404:15–21.https://doi.org/10.1042/BJ20061489
-
Double hearts produced experimentally in rat embryosJournal of Experimental Zoology 72:33–49.https://doi.org/10.1002/jez.1400720103
-
Filopodia as sensorsCellular Signalling 25:2298–2311.https://doi.org/10.1016/j.cellsig.2013.07.006
-
Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinaseThe Journal of Biological Chemistry 280:26089–26093.https://doi.org/10.1074/jbc.M504045200
-
Platelet-derived growth factor receptor-α is essential for cardiac fibroblast survivalAmerican Journal of Physiology. Heart and Circulatory Physiology 317:H330–H344.https://doi.org/10.1152/ajpheart.00054.2019
-
Classes of phosphoinositide 3-kinases at a glanceJournal of Cell Science 127:923–928.https://doi.org/10.1242/jcs.093773
-
Molecular regulation of pancreas development in zebrafishMethods in Cell Biology 100:261–280.https://doi.org/10.1016/B978-0-12-384892-5.00010-4
-
Phospholipids of the plasma membrane - regulators or consequence of cell polarity?Frontiers in Cell and Developmental Biology 8:277.https://doi.org/10.3389/fcell.2020.00277
-
The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructsDevelopmental Dynamics 236:3088–3099.https://doi.org/10.1002/dvdy.21343
-
Using live imaging to examine early cardiac development in zebrafishMethods in Molecular Biology 2438:133–145.https://doi.org/10.1007/978-1-0716-2035-9_9
-
Methods for cell and particle trackingMethods in Enzymology 504:183–200.https://doi.org/10.1016/B978-0-12-391857-4.00009-4
-
Maternal or zygotic sphingosine kinase is required to regulate zebrafish cardiogenesisDevelopmental Dynamics 244:948–954.https://doi.org/10.1002/dvdy.24288
-
Bidirectional fusion of the heart-forming fields in the developing chick embryoDevelopmental Dynamics 235:191–202.https://doi.org/10.1002/dvdy.20601
-
Sample drift correction following 4D confocal time-lapse imagingJournal of Visualized Experiments 1:51086.https://doi.org/10.3791/51086
-
Proteomics analysis of early developmental stages of zebrafish embryosInternational Journal of Molecular Sciences 20:6359.https://doi.org/10.3390/ijms20246359
-
Roles of leader and follower cells in collective cell migrationMolecular Biology of the Cell 32:1267–1272.https://doi.org/10.1091/mbc.E20-10-0681
-
A study in the localization of organ-forming areas in the chick blastoderm of the head-process stageJournal of Experimental Zoology 72:271–315.https://doi.org/10.1002/jez.1400720205
-
FGF8 signaling is chemotactic for cardiac neural crest cellsDevelopmental Biology 354:18–30.https://doi.org/10.1016/j.ydbio.2011.03.010
-
Collective cell migration in developmentAdvances in Experimental Medicine and Biology 1146:105–116.https://doi.org/10.1007/978-3-030-17593-1_7
-
Using zebrafish to analyze the genetic and environmental etiologies of congenital heart defectsAdvances in Experimental Medicine and Biology 1236:189–223.https://doi.org/10.1007/978-981-15-2389-2_8
-
Detection of damage-activated metacaspase activity by western blot in plantsMethods in Molecular Biology 2447:127–137.https://doi.org/10.1007/978-1-0716-2079-3_11
-
Myocardialization of the cardiac outflow tractDevelopmental Biology 212:477–490.https://doi.org/10.1006/dbio.1999.9366
-
A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002)The Journal of Biological Chemistry 269:5241–5248.
-
Somatic mutations of PTEN in glioblastoma multiformeCancer Research 57:4183–4186.
-
The migration of heart mesoderm and associated areas in Amblystoma punctatumJournal of Experimental Zoology 129:579–605.https://doi.org/10.1002/jez.1401290308
Decision letter
-
Richard P HarveyReviewing Editor; Victor Chang Cardiac Research Institute, Australia
-
Didier YR StainierSenior Editor; Max Planck Institute for Heart and Lung Research, Germany
-
Osvaldo ContrerasReviewer; Victor Chang Cardiac Research Institute, Australia
Our editorial process produces two outputs: (i) public reviews designed to be posted alongside the preprint for the benefit of readers; (ii) feedback on the manuscript for the authors, including requests for revisions, shown below. We also include an acceptance summary that explains what the editors found interesting or important about the work.
Decision letter after peer review:
Thank you for submitting your article "The myocardium utilizes Pdgfra-PI3K signaling to steer towards the midline during heart tube formation" for consideration by eLife. Your article has been reviewed by 3 peer reviewers, one of whom is a member of our board of Reviewing Editors, and the evaluation has been overseen by Didier Stainier as the Senior Editor. The following individual involved in the review of your submission has agreed to reveal their identity: Osvaldo Contreras (Reviewer #2).
The reviewers have discussed their reviews with one another, and the Reviewing Editor has drafted this to help you prepare a revised submission.
Essential revisions:
The reviewers found this to be a valuable contribution as a Research Advance – however, significant weaknesses and discrepancies were identified. The authors therefore should respond to all of the reviewers' comments. Reviewers have asked for additional data to support current findings and to extend the model of PDGFRa/PI3K-mediated chemotactic migration of cardiomyocyte precursors during heart tube formation in zebrafish. We consider essential revisions to be:
1) Address spatially PI3K activity in vivo to further address the model and to confirm and understand the impact of PI3K inhibition using drugs, and the dominant negative reagent.
2) Address the discrepancies between single cardiomyocyte trajectory data in the original and current work, and repeat more rigorously if necessary. Direct the research towards understanding the different behaviors evident in anterior, medial, and posterior zones, and align with the model.
3) Strengthen experimentally the observations around cardiomyocyte protrusions, including experiments that would extend the model by distinguishing between individual cell versus collective cell migration. If possible, address whether protrusions are involved in cell steering.
4) It would be a strong addition to the study if the authors could show rescue of PI3K inhibition (with drugs and/or dominant negative transgene) with the Pdgf-aa transgene used in the previous study and in the current study to support PI3K/pAKT being downstream of PDGFRA.
5) Please include the zebrafish model in the title as per guidelines and please spell out PDGFRa and PI3K.
Reviewer #1 (Recommendations for the authors):
The manuscript by Shrestha and Bloomekatz and colleagues addresses the role of PI3K signaling in latero-medial migration of committed cardiomyocyte cell progenitor populations during the formation of the early heart tube in zebrafish development, disruption of which causes complete or partial cardia bifida. The work is submitted as a Research Advance, which builds on a previous Research Article in eLife (Bloomekatz et al., eLife 2017;6:e21172). In the previous paper, Bloomekatz and colleagues reported the isolation and analyses of the ref mutation, showing that it interrupts the function of the Pdgfra gene. They go on to characterise defects in the medial migration of cardiac progenitors in the genetic mutant and also in pdgfra morpholino knockdown experiments. Pdgfra is expressed in the anterior lateral mesoderm during migration and fusion, whereas its ligand Pdgfaa is expressed in the anterior endoderm, known to be important for cardiomyocyte fusion, potentially for physically propelling (via extracellular matrix) the cardiac mesoderm along. Live imaging and quantification showed that the Pdgfra expression in cardiac mesoderm is required for directional movement (steering) of cardiomyocytes to the midline during heart tube formation, presumably via a chemotactic mechanism involving graded signals from endoderm. Effects on velocity and efficiency of movement of cardiomyocytes were not evident.
In the present manuscript, Bloomekatz and colleagues explore the role of PI3K in latero-medial cardiomyocyte migration in zebrafish, potentially working downstream of Pdgfra. A similar overall approach is taken as in the first paper, albeit that the PI3K is inhibited using the drug LY294002 (confirmed using other classes of inhibitor), or cardiomyocyte-specific transgenic expression of a truncated dominant negative form of PI3K. In terms of prior knowledge, and therefore the novelty of this paper, the PI3K pathway is known to function downstream of Pdgfr signaling and indeed in migration and proliferation of Pdgf-dependent cells and other cell types in the development, including in collective cell migrations. PI3K is also involved in the formation of cell protrusions in some settings, and cardiomyocyte protrusions have been observed in developing mouse hearts. The manuscript is transparent on background studies; however, involvement of PI3K is therefore somewhat expected. Nonetheless, the authors show convincingly that PI3K inhibition affects cardiomyocyte latero-medial migration in a dose-dependent manner. They define a broad window for the involvement of PI3K during the migration process and show that cardiomyocyte-specific expression of dominant negative PI3K causes migration defects in 100% of embryos, demonstrating that this is a cell-autonomous process. Similar to the original study, live imaging with a myl7-eGFP transgene enabled the authors to quantify defects in individual cardiomyocyte trajectories and determine average scores for velocity, efficiency, and direction of movement, which were all compromised after PI3K inhibition. Elements of cell behaviour at different time points along the migration process were considered. As a potential mechanism, they demonstrate cellular protrusions in migrating cardiomyocytes and quantify their number, duration, and direction relative to the axis of migration. An important finding is that these protrusions are oriented towards the direction of migration in control embryos, but this is lost after PI3K inhibition. Finally, an interaction between Pdgfra heterozygosity and PI3K inhibition is demonstrated, suggesting that PI3K does function downstream of Pdgfra signaling.
Recommendations:
1. Inhibition of PI3K might lead to phenotypes that are weaker (partial inhibition) or stronger (inhibition of PI3K in tissues beyond cardiomyocytes); however, one would expect given the results of the cardiomyocyte-specific dnPI3K transgene, that the phenotypes of Pdgfra ref mutants and PI3K inhibition would closely align. One significant concern, therefore, is that the quantifications of cell trajectories look very different comparing Bloomekatz et al. 2017 to the current study. In the original study, control cardiomyocytes have much broader angles of trajectory compared to the very limited angles in the current study. Similarly, ref mutant cardiomyocytes often migrate in alternate directions along the latero-medial axis and sometimes in a net backward direction, not seen in the current study with PI3K inhibition. Comparing the trajectories in the current paper, it appears that they are mostly latero-medial in orientation anteriorly, whereas those occurring posteriorly have a distinct anterior-medial direction. These could be driven by different processes. Visual inspection of the trajectories in the inhibited embryos seems to show that the posterior type is preserved, whereas the anterior type is diminished. The authors have perhaps lost the opportunity to segment classes of behavior to refine the model of cardiomyocyte chemotactic morphogenesis. The authors do present segmentation of anterior versus posterior behaviors; however, it seems that the posterior ones differ between the control and mutant, not the anterior ones, which might go against the visual inspection of panels in both Figure 3B, D, and Suppl. Figure 5D. At a minimum, the authors should address the differences between the two studies – are these differences a product of methodology or normalisation (e.g. of notochord contraction) or to features of the gene model, if so which? and how does this relate to the model of migration? It would help to better relate trajectory findings to literature models.
2. It would strengthen the paper to show spatially PI3K activity across the migration period. Phospho-ATK has been used previously to show receptor tyrosine kinase activity in vivo in different settings (as shown in this study by western blotting).
3. The characterization of cardiomyocyte epithelial morphology is cursory, and I think other markers of epithelial morphology, apical-basal polarity or integrity could be shown, perhaps N-CAM. The authors should be more specific in their description of the processes leading to the second dorsal layer (which is currently vague and seems not visible in the control sample so panels are not comparable).
4. Are the protrusions at the leading edge of the cardiomyocyte population or throughout? Does this instruct on how migration occurs – as individual cells in a partial mesenchymal state, or as a sheet?
5. The tools used to explore endodermal integrity (gross expression of axial and Sox17-gfp) seem inadequate. The question as to whether endoderm is compromised is paramount, as endoderm has been proposed to instruct cardiomyocyte migration, likely by the provision of chemotactic ligands (e.g. Pdgf-aa), however, potentially also by physical means. Is its own migration relative to that of cardiomyocytes compromised? Knowing this could advance the model.
6. Page 6. Examples of control versus inhibited embryos stage-matched for somite number should be shown in Suppl. Figure 1. I don't see any reference to somite stage matching in Suppl. Figure 2, only that somite number is retarded in inhibited embryos.
7. Please state in the manuscript and/or show in a table the known specificities of the inhibitors used.
8. I think the statistics for the cell behavioural studies should be shown on the graphs in a more conventional way.
9. It would be helpful to cite other examples where cardiomyocyte protrusions have been described; for example, dedifferentiated neonatal cardiomyocytes in vitro (Eldad Tzahor lab), regenerating scar after myocardial infarction (James Martin lab) and myocardialization of outflow track cushions (Antoon Moorman lab).
Reviewer #2 (Recommendations for the authors):
Recommendations:
1. The authors should refer to their concentrations used in the results field (Page 6). I also think that the concentrations used are higher than expected (especially for Dactolisib and Pictilisib). Thus, the authors should be extremely cautious about their results and their interpretations since off-target effects could potentially target myocardial fusion. I had a quick look and the IC50 of Pictilisib is in nM range and the authors used 50µM, which is 1000-fold higher.
2. I suggest the authors extend their evaluation of PI3K downstream targets including GSK3B and mTOR. This aims to better understand the mechanisms associated with PI3K pharmacological inhibition.
3. The authors mention "Furthermore, the PI3K signaling pathway is known to promote cell proliferation and cell survival (29) however, we did not find a difference in the number of cardiomyocytes in DMSO- or LY- treated embryos at 20s (Suppl. Figure 3G-I). I suggest the authors evaluate not only the total cardiomyocyte number but cardiomyocyte cell cycle and/or proliferation, using techniques such as BrdU/EdU uptake, Ki67 labelling, and phosphorylated Histone H3 in Serine 10. Equal cardiomyocyte numbers do not necessarily point to no defects in the cardiomyocyte cell cycle and proliferation potentially induced by PI3K inhibitors (known to affect the cell cycle in many different cell types and contexts).
4. The authors refer to cardiomyocyte apoptosis on Page 7 but no figure reference exists. Please, check and add the missing results.
5. Line 19, page 8. The authors refer to myocardial movement, but they have not directly addressed that point yet. I suggest the authors amend the sentence. The same applies to line 10, page 10.
6. Line 1, page 10. I would change "indicating" to "suggesting".
7. Would the increased PDGFRa activity obtained using mutant zebrafish over-expressing Pdgf-aa rescue PI3K-inhibited embryos? I think this is an important point to be addressed.
8. Page 16, line 14. The authors refer to "protrusion formation" but if I understand well, they never evaluated that later cellular effect.
Recommendations related to Figures and related conclusions:
1. Immunofluorescence against phosphorylated AKT during cardiac fusion (using Tg(myl7:egfp zebrafish)) could strengthen the authors' results.
2. The authors should evaluate PI3K activity (AKT phosphorylation) in the case of Dactolisib and Pictilisib inhibitors. dnPI3K would also be highly appreciated.
3. In order to explain the PI3K-mediated defects during cardiac fusion, I would change the order of Figure 4 by Figure 3. Thus, PI3K-induced defects on myocardial membrane protrusions may better explain the resultant medial movement and myocardium speed during cardiac fusion. It reads better from my point of view in that way.
4. The authors should quantify the number of protrusions per cell and not only the direction. If the number of protrusions is reduced that should open new avenues related to the coordinated migratory mechanisms during cardiac fusion, but if the numbers do not change that may reinforce the authors conclusions.
5. Figure 5F. The results of 10µM LY inhibitor in Pdgfra +/+ embryos do not match well with Supplementary Figure 1M. Please check and discuss if necessary.
6. Do the authors observe a genetic interaction between dnPI3K and Pdgfras16 mutants (het or homo)?
7. Figure 5. Western blots for p-AKT are based on total embryo lysates. Thus, it is expected to find what the authors found related to Pdgfra mutants and hs:pdgfaa. The manuscript would be strengthened if the authors could provide direct evidence of phosphorylated AKT in the myocardial region during cardiac fusion. The authors could use immunofluorescence and confocal imaging.
8. I am a bit conflicted with Supplementary Figure 5C. What each dot represents? A single cell? I suggest the authors read the latest thread about some important statistical considerations:
https://doi.org/10.1083/jcb.202001064
https://rupress.org/jcb/article/219/6/e202001064/151717/SuperPlots-Communicating-reproducibility-and
Reviewer #3 (Recommendations for the authors):
Overall, the manuscript is well written and experiments are well controlled providing sufficient evidence to substantiate most of their conclusions. There are a number of questions that need to be addressed by the authors.
Specific points:
1. What is interesting is that the CPCs have some intrinsic migration that is independent of the endoderm. The finding that Pi3K is required for the cardiac fusion process is not too surprising as the authors showed in their previous work that PDGF signaling is driving this process and PI3K is a well-known transducer of PDGF signaling. An important question remaining is what type of cell migration the CPCs undergo to fuse at the midline. Are the CPCs migrating as individual cells that all respond to the PDGF from the midline or do the CPCs migrate collectively and only a few respond to the PDGF signal? The authors seem to prefer the collective migration of CPCs but it seems that this has not been tested experimentally. Testing this directly would significantly increase the impact of this study. With the myl7:dnPI3K construct the authors seem to have a good tool to test this now. This could be addressed by analysing embryos in which the cardiac field contains a mixture of wt CPCs and CPCs expressing the dnPIK3 for example by cell transplantation experiments in which cells from embryos derived from the myl7:dnPI3K F1 fish are transplanted to wt embryos or by DNA injections resulting in mosaic expression. It would be very interesting to see how CPCs expressing the dnPI3K behave when surrounded by wt cells. If collective migration is indeed driving cardiac fusion one would expect that these dnPI3K cells behave like wt cells.
2. In Figure 3 the authors show that migration of CPCs treated with LY is affected. They plotted the CPC velocities in 3E and here it looks as if fast-migrating CPCs are less affected compared to slower-migrating CPCs. Fast migration is mostly seen in the posterior part of the bilateral heart fields, so the question arises whether CPCs along the anterior-posterior axis is affected differently by the LY treatment.
3. In fig3E the authors show the total velocity of the CPCs in all directions. As they conclude at the end that PDGF-PI3K signaling mostly affects the medial cell migration, it would be important to analyse the velocities of the CPCs only in the medial-lateral direction.
4. In Figure 3G the authors show the angles at which the CPCs migrate based on the time lapses. In Figure 3B the tracks show that some cells (mostly located anteriorly) move in a medial-posterior direction while other cells (mostly located in the posterior part) move in an anterior-medial direction. The rose plot however displays only positive angles (medial-anterior direction) and no angles in the medial-posterior direction. Please explain why this was done. In addition, the angles shown here for wt embryos (average 30 degrees) seem very different from the angles reported for wt embryos in their previous work (Fig6L, average around 60 degrees), which look more like the angles reported here for the LY treated embryos (average around 60 degrees). Please explain this.
5. In Figure 4 the authors analyse the direction and persistence of cellular protrusion in CPCs. Especially the direction of these protrusions seems affected by LY suggesting that PDGF from the midline may be responsible for this phenomenon. It would therefore be very interesting to test this in their hs:pdgf line by analysing protrusion direction when pdgf is produced by all cells. If PDGF from the midline is responsible for their direction, overexpressing pdgf in all cells should give a similar effect as inhibiting PI3K.
https://doi.org/10.7554/eLife.85930.sa1Author response
Essential revisions:
The reviewers found this to be a valuable contribution as a Research Advance – however, significant weaknesses and discrepancies were identified. The authors therefore should respond to all of the reviewers' comments. Reviewers have asked for additional data to support current findings and to extend the model of PDGFRa/PI3K-mediated chemotactic migration of cardiomyocyte precursors during heart tube formation in zebrafish. We consider essential revisions to be:
1) Address spatially PI3K activity in vivo to further address the model and to confirm and understand the impact of PI3K inhibition using drugs, and the dominant negative reagent.
We thank the reviewers for this suggestion. We agree that this is an important issue. We’ve addressed the issue of tissue-specificity of PI3K activity in a couple different ways. For example, by expressing a dominant negative construct specifically in the myocardium using the myl7 promoter (Figure 2C-H), we showed that PI3K signaling is specifically required within myocardial cells to regulate cardiac fusion.
We have further addressed this question in our revisions by employing a pleckstrin homology (PH)-domain PIP3 reporter, Tg(myl7:PH-mkate2). PH domains from several proteins including from BTK have been shown to translocate to the membrane in response to PI3K-induced PIP3 production (Hall, TE et al. 2020, PMID: 32709891; Yoo, SK et al. 2010, PMID: 20159593). In DMSO-treated embryos, we observed PH-mkate2 localize to the plasma membrane of myocardial cells, indicating PI3K activity. Conversely, we found that the PH-mkate2 in the cytoplasm or in sub-cellular compartments in PI3K-inhibited embryos. A detailed analysis of these new results is contained within Figure 2 —figure supplement 2 as well as to changes in the text (pages 11-12, lines 19-22, 1-6, respectively).
2) Address the discrepancies between single cardiomyocyte trajectory data in the original and current work, and repeat more rigorously if necessary. Direct the research towards understanding the different behaviors evident in anterior, medial, and posterior zones, and align with the model.
We thank the reviewers for their close reading of our manuscript and the literature. In our study, myocardial cell trajectories in DMSO-treated embryos are initially dominated by medial-lateral directionality and then by more angular movement (anterior-posterior directionality) (Figure 3A-B). These observations are overall consistent with findings from both the original work (Bloomekatz et al. 2017) and from other previously published studies (Holtzman et al. 2007). As suggested by the reviewers, we have increased our analysis of myocardial movements in this manuscript separating them by developmental stage (early, posterior, anterior) and by anterior, medial and posterior zones (Figure 3 —figure supplement 2C-F). These analyses reaffirm our qualitative observations of increasing angular movements during later developmental stages and of relative increases in angular velocity in anterior and posterior cells.
In this study a greater percentage of tracks are medially directed (Figure 3G) when compared to Bloomekatz et al. 2017 – Figure 6L. These minor differences are likely a result of technical differences in experimentation, including normalization using the notochord and differences in the developmental stages captured by the timelapse data. Variation between could also contribute these differences. We have incorporated these considerations into our revised discussion (page 18, lines 16-20).
3) Strengthen experimentally the observations around cardiomyocyte protrusions, including experiments that would extend the model by distinguishing between individual cell versus collective cell migration. If possible, address whether protrusions are involved in cell steering.
We share the reviewers’ enthusiasm for our observations of cardiomyocyte protrusions. In our studies we identified myocardial protrusions during cardiac fusion which are oriented in the medial-lateral direction in DMSO-treated embryos but are mis-oriented in PI3K-inhibited embryos. In our revisions we have strengthened our observations of these myocardial protrusions, as suggested by the reviewer. Through these enhanced observations we identified different protrusion types: thin protrusions similar to filopodia and wide protrusions similar to pseudopodia. And we analyzed how the ratio and orientation of these different protrusions changes in LY-treated (Figure 4—figure supplement 1).
We do agree with the reviewers regarding the value of further experiments regarding the overall role for protrusions in cardiomyocyte movement and their role in the collective movement of the myocardium. However, these experiments are distinct from our current study focusing on PI3K signaling and its connection to Pdgfra. We thus plan to include them in a more comprehensive study of the role of cardiomyocyte protrusions in cardiac fusion in the future.
4) It would be a strong addition to the study if the authors could show rescue of PI3K inhibition (with drugs and/or dominant negative transgene) with the Pdgf-aa transgene used in the previous study and in the current study to support PI3K/pAKT being downstream of PDGFRA.
In response to valuable reviewer feedback regarding strengthening our finding of an interaction between Pdgfra and PI3K signaling during cardiac fusion, we investigated if the cardiac fusion defects found in embryos overexpressing pdgfaa could be rescued by using a sub-phenotypic dose of LY. We found that while 55.5% of heat-shocked Tg(hs:pdgfaa) embryos exposed to DMSO display cardiac fusion defects, only 17% of heat-shocked Tg(hs:pdgfaa) embryos exposed to 10µM LY showed cardiac fusion defects (p-value 0.0184) (Figure 5G-J). This result complements our previous findings of genetic interaction between pdgfra -/+ and 10µM LY exposure (Figure 5C-F) and that PI3K activity is regulated by changes in pdgfra activity (both loss- and gain- offunction) (Figure 5A, B). Together these experiments suggest that Pdgfra and PI3K signaling work together to regulate cardiac fusion. These changes have been incorporated into Figure 5 and page 16, lines 3-5.
5) Please include the zebrafish model in the title as per guidelines and please spell out PDGFRa and PI3K.
Thank you for pointing out this oversight, it has been corrected.
Reviewer #1 (Recommendations for the authors):
Recommendations:
1. Inhibition of PI3K might lead to phenotypes that are weaker (partial inhibition) or stronger (inhibition of PI3K in tissues beyond cardiomyocytes); however, one would expect given the results of the cardiomyocyte-specific dnPI3K transgene, that the phenotypes of Pdgfra ref mutants and PI3K inhibition would closely align. One significant concern, therefore, is that the quantifications of cell trajectories look very different comparing Bloomekatz et al. 2017 to the current study. In the original study, control cardiomyocytes have much broader angles of trajectory compared to the very limited angles in the current study. Similarly, ref mutant cardiomyocytes often migrate in alternate directions along the latero-medial axis and sometimes in a net backward direction, not seen in the current study with PI3K inhibition. Comparing the trajectories in the current paper, it appears that they are mostly latero-medial in orientation anteriorly, whereas those occurring posteriorly have a distinct anterior-medial direction. These could be driven by different processes. Visual inspection of the trajectories in the inhibited embryos seems to show that the posterior type is preserved, whereas the anterior type is diminished. The authors have perhaps lost the opportunity to segment classes of behavior to refine the model of cardiomyocyte chemotactic morphogenesis. The authors do present segmentation of anterior versus posterior behaviors; however, it seems that the posterior ones differ between the control and mutant, not the anterior ones, which might go against the visual inspection of panels in both Figure 3B, D, and Suppl. Figure 5D. At a minimum, the authors should address the differences between the two studies – are these differences a product of methodology or normalisation (e.g. of notochord contraction) or to features of the gene model, if so which? and how does this relate to the model of migration? It would help to better relate trajectory findings to literature models.
We thank the reviewer for their detailed reading of our manuscript and our previous work. As we outlined in our response to essential revisions #2, we have increased our analysis of cardiomyocyte trajectories in wild-type/DMSO-treated embryos (Figure 3 —figure supplement 2C-F). Overall, our data is consistent both with our previous work and with the work of others (Holtzman et al. 2007, PMID: 17537802). The main difference between these studies resides in the rose plots of wild-type myocardial trajectories in Bloomekatz et al. 2017 and the DMSO-trajectories in this manuscript. This difference is likely due to experimental variation in the developmental stages over which the timelapse data were collected, differences in normalization to the notochord, and/or differences reflective of variance between embryos. We now include a discussion of these differences in manuscript (page 18, lines 16-20).
In regards to comparisons of cell trajectory between ref/pdgfra mutant and PI3K mutants; there are strong similarities between these trajectories including defects in medial-lateral directionality, speed and movement defects being visible at the start of the timelapse movies. However, as the reviewer notes there are also differences between these trajectories, with the ref/pdgfra mutant myocardial cells in a few embryos displaying lateral movement that is not present in PI3K-inhibited embryos. These differences could be due to extent of pdgfra inhibition compared to PI3K inhibition. Furthermore, other intracellular signaling pathways parallel to PI3K signaling and downstream of Pdgfra, such as MAPK and PLCg could play minor compensatory roles in steering myocardial movement, thus explaining the less severe result in PI3Kinhibited embryos. We have increased our discussion of these considerations in the revised manuscript (page 19, lines 2 2-8).
The reviewer also mentions examining whether locations in cardiac ring (anterior vs posterior) are differently affected. We have now included this analysis in Figure 3 —figure supplement 2.
2. It would strengthen the paper to show spatially PI3K activity across the migration period. Phospho-ATK has been used previously to show receptor tyrosine kinase activity in vivo in different settings (as shown in this study by western blotting).
We agree with the reviewer that the issue of spatial PI3K activity/tissue-specificity is an important one. As we have highlighted in our response to essential revision #1 – we have addressed this issue in a number of ways including myocardial specific inhibition of PI3K activity (Figure 2). In our revisions we have now included experiments involving a PI3K activity PH-domain reporter (Figure 2 —figure supplement 2) in which we confirm PI3K activity in the myocardium during the later stages of cardiac fusion. In the future we plan to extend these studies to all stages of cardiac fusion.
3. The characterization of cardiomyocyte epithelial morphology is cursory, and I think other markers of epithelial morphology, apical-basal polarity or integrity could be shown, perhaps N-CAM. The authors should be more specific in their description of the processes leading to the second dorsal layer (which is currently vague and seems not visible in the control sample so panels are not comparable).
We thank the reviewer for their comment. We have modified Figure 1—figure supplement 4 to indicate the location of the second dorsal layer (arrows), which has been previously noted (Trinh, LA et al. 2004, PMID: 15030760; Ye, D et al. 2015, PMID: 26329600). And we have clarified that this process occurs around the same time myocardial cells move ventral to the endoderm, a process that has been termed subduction (pages 7-8, lines 21-22, 1-2, respectively), although the cellular and molecular processes that underlie this formation have not been fully elucidated.
4. Are the protrusions at the leading edge of the cardiomyocyte population or throughout? Does this instruct on how migration occurs – as individual cells in a partial mesenchymal state, or as a sheet?
We agree with the reviewer, an examination of how protrusions change in different regions of the myocardial populations and at different developmental timepoints is an interesting question. In our revisions we have enhanced our analysis of cardiomyocyte protrusions by identifying protrusions with different morphologies. In our future work, we hope to build on these studies including addressing the role of these protrusions in migration.
5. The tools used to explore endodermal integrity (gross expression of axial and Sox17-gfp) seem inadequate. The question as to whether endoderm is compromised is paramount, as endoderm has been proposed to instruct cardiomyocyte migration, likely by the provision of chemotactic ligands (e.g. Pdgf-aa), however, potentially also by physical means. Is its own migration relative to that of cardiomyocytes compromised? Knowing this could advance the model.
We appreciate Reviewer 1’s comment regarding investigating endodermal integrity during cardiomyocyte migration. In response to this feedback, we have now added high resolution confocal images of the endoderm in DMSO-treated and PI3K-inhibited embryos to support our conclusion that the morphology of the endoderm is not compromised in PI3K-inhibited embryos (Figure 2 —figure supplement 1I, J).
6. Page 6. Examples of control versus inhibited embryos stage-matched for somite number should be shown in Suppl. Figure 1. I don't see any reference to somite stage matching in Suppl. Figure 2, only that somite number is retarded in inhibited embryos.
All the embryos shown in the figures, including in Suppl. Figure 1 (now – Figure 1 —figure supplement 1) are stage matched by somite number, except the embryos in Suppl. Figure 2 (now – Figure 1 —figure supplement 2) which are matched by time postfertizilation (hpf). We have modified the text (page 6, lines 13-17) to clarify this fact.
7. Please state in the manuscript and/or show in a table the known specificities of the inhibitors used.
We thank the reviewer for their suggestion and we have now included statements and references to known off-targets of each of the inhibitor (page 7, lines 6-8). They are also included in Author response table 1.
| Inhibitors | Target | Known off-targets |
|---|---|---|
| LY294002 | Class I PI3Ks | mTOR, DNA-PK |
| Dactolisib | Class I PI3Ks, mTOR | |
| Pictilisib | Class I PI3Ks | mTOR |
| Rapamycin | mTOR | |
| VOOH | Pten |
References: Maira, SM. et al. 2008 PMID: 18606717; Folkes, A. et al. 2008 PMID:
18754654; Rosivatz, E. et al. 2006 PMID: 17240976, Gharbi, S.I. et al. 2007 PMID:
17302559.
8. I think the statistics for the cell behavioural studies should be shown on the graphs in a more conventional way.
We had previously represented variation in our graphs by showing all individual cells, and displaying a box-whisker plot, which shows the median, the location of the quartiles and the min and max. As suggested by the reviewers we have now switched the graphs in the cell behavioral studies to superplots (Lord, S. et al. 2020 PMID: 32346721) in which all cells are shown along with the averages from individual embryos, and the mean and standard error are shown along with data from the appropriate statistical test, see Figures 3E-H, Figure 3 —figure supplement 2C-F.
9. It would be helpful to cite other examples where cardiomyocyte protrusions have been described; for example, dedifferentiated neonatal cardiomyocytes in vitro (Eldad Tzahor lab), regenerating scar after myocardial infarction (James Martin lab) and myocardialization of outflow track cushions (Antoon Moorman lab).
We thank the reviewer for alerting us to this oversight, these citations as well as those for myocardial protrusions occurring during cardiac trabeculation are now included in the manuscript (page 19, lines 17-19).
Reviewer #2 (Recommendations for the authors):
Recommendations:
1. The authors should refer to their concentrations used in the results field (Page 6). I also think that the concentrations used are higher than expected (especially for Dactolisib and Pictilisib). Thus, the authors should be extremely cautious about their results and their interpretations since off-target effects could potentially target myocardial fusion. I had a quick look and the IC50 of Pictilisib is in nM range and the authors used 50µM, which is 1000-fold higher.
We share the reviewers concern regarding using high concentrations of inhibitor. However, the reported nanomolar IC50 values are determined using cell-free assays, and may not account for issues of stability, metabolism, penetrance, among others that are encountered in live animal studies. Indeed, our concentrations fall within the range of concentrations used in other live animal studies (Montero, JA et al. 2003, PMID: 12906787; Sasore, T. et al. 2014, PMID: 25144531; Junttila, T. et al. 2009, PMID: 19411071).
2. I suggest the authors extend their evaluation of PI3K downstream targets including GSK3B and mTOR. This aims to better understand the mechanisms associated with PI3K pharmacological inhibition.
We thank Reviewer 2 for this valuable feedback. In response, we investigated if mTOR is required for cardiac fusion by exposing embryos to increasing concentrations of Rapamycin. We did not observe any cardiac fusion defects in embryos when mTOR was inhibited, even at very high concentrations, as shown in Figure 1 —figure supplement 3A-F.
3. The authors mention "Furthermore, the PI3K signaling pathway is known to promote cell proliferation and cell survival (29) however, we did not find a difference in the number of cardiomyocytes in DMSO- or LY- treated embryos at 20s (Suppl. Figure 3G-I). I suggest the authors evaluate not only the total cardiomyocyte number but cardiomyocyte cell cycle and/or proliferation, using techniques such as BrdU/EdU uptake, Ki67 labelling, and phosphorylated Histone H3 in Serine 10. Equal cardiomyocyte numbers do not necessarily point to no defects in the cardiomyocyte cell cycle and proliferation potentially induced by PI3K inhibitors (known to affect the cell cycle in many different cell types and contexts).
We appreciate Reviewer 2’s suggestions and we have now included an experiment investigating the number of cardiomyocytes in S phase by analyzing EdU uptake. We did not find a statistical difference between in DMSO and PI3K inhibited embryos. These findings are reported in Figure 1 —figure supplement 4.
4. The authors refer to cardiomyocyte apoptosis on Page 7 but no figure reference exists. Please, check and add the missing results.
We have now added images (Figure 1 —figure supplement 4M-P) and quantitative data (Figure 1 —figure supplement 4 source data), showing no TUNEL+ cardiomyocytes in DMSO- nor in LY-treated embryos, even though TUNEL+ cells are evident when embryos are treated with DNAase.
5. Line 19, page 8. The authors refer to myocardial movement, but they have not directly addressed that point yet. I suggest the authors amend the sentence. The same applies to line 10, page 10.
We have changed movement to translocation (page 10, line 12; page 12, line 17) as requested by the reviewer.
6. Line 1, page 10. I would change "indicating" to "suggesting".
We have made the suggested change (page 12, line 7), thank you.
7. Would the increased PDGFRa activity obtained using mutant zebrafish over-expressing Pdgf-aa rescue PI3K-inhibited embryos? I think this is an important point to be addressed.
We thank Reviewer 2 for their suggestion. During our revisions, we performed this experiment and found that a sub-phenotypic reduction in PI3K activity could rescue the cardiac fusion defects caused by overexpressing Pdgf-aa. This result is reported in Figure 5G – J and in the manuscript (page 16, lines 3-5).
8. Page 16, line 14. The authors refer to "protrusion formation" but if I understand well, they never evaluated that later cellular effect.
Thank you for pointing out this oversight. We have now modified the text (page 17, line 9).
Recommendations related to Figures and related conclusions:
1. Immunofluorescence against phosphorylated AKT during cardiac fusion (using Tg(myl7:egfp zebrafish)) could strengthen the authors' results.
We appreciate the reviewer’s suggestion. However, despite our diligent efforts we have been unable to reliably achieve a successful immunofluorescence staining in zebrafish for phosphorylated Akt (pAkt). As an alternative approach, we employed a pleckstrin homology (PH) domain reporter – PH-mkate2, to assess PI3K activation during cardiac fusion. PH-domains that bind to PIP3 are localized to the membrane when PIP3 is present. Our observations revealed PH-mkate2 at the plasma membrane of cardiomyocytes in DMSO-treated embryos, but not in LY-treated embryos. This information is now included in our revised manuscript (see Figure 2 —figure supplement 2 and pages 11-12, lines 19-22, lines 1-6, respectively).
2. The authors should evaluate PI3K activity (AKT phosphorylation) in the case of Dactolisib and Pictilisib inhibitors. dnPI3K would also be highly appreciated.
We thank the reviewer for the suggestion and apologize for this oversight. We have now evaluated pAKT in comparison to Akt levels in embryos treated with Dactolisib, Pictilisib and dnPI3K. We observed a dose-dependent decrease in PI3K activity as reported by pAKT-to-AKT levels. This data is now included in our revised manuscript (Figure 1 —figure supplement 1Q-S, and on page 7, lines 1-5, 15).
3. In order to explain the PI3K-mediated defects during cardiac fusion, I would change the order of Figure 4 by Figure 3. Thus, PI3K-induced defects on myocardial membrane protrusions may better explain the resultant medial movement and myocardium speed during cardiac fusion. It reads better from my point of view in that way.
We thank the reviewer for this suggestion – having revised the manuscript in this order; the logical flow felt off and so we have changed it back. We hope our other revisions have increased the logical flow for the readers.
4. The authors should quantify the number of protrusions per cell and not only the direction. If the number of protrusions is reduced that should open new avenues related to the coordinated migratory mechanisms during cardiac fusion, but if the numbers do not change that may reinforce the authors conclusions.
We appreciate the reviewer’s suggestion. In response to this query, we quantified the frequency of protrusions per cell per hour and found that cardiomyocytes in DMSOtreated embryos display an average of 20.3 -/+ 6.7 protrusions per hour, while cardiomyocytes in LY-treated embryos display an average of 17.00 -/+ 7.4 protrusions per hour (p-value: 0.3562, Two-tailed t-test), indicating no significant difference between the frequency of protrusions. (This data is now included in our manuscript, page 14 lines 11-16 and in Figure 4-source data.)
5. Figure 5F. The results of 10µM LY inhibitor in Pdgfra +/+ embryos do not match well with Supplementary Figure 1M. Please check and discuss if necessary.
We appreciate the reviewer’s careful reading of our manuscript. In Figure 5F and Figure 1 —figure supplement 1M, separate experiments were conducted involving 10 µM inhibitor treatments. Although, there are slight differences in percent of embryos displaying cardiac phenotypes (None in Figure 1 —figure supplement 1M, compared to an average of 10.9% in Figure 5C), these differences are not statistically significant (pvalue: 0.2784, Two-tailed t-test). The minor variation from these distinct experiments could have arisen from the different genetic backgrounds of the fish used in this experiment or from differences in the batches of inhibitor.
6. Do the authors observe a genetic interaction between dnPI3K and Pdgfras16 mutants (het or homo)?
We appreciate the reviewer’s suggestion, unfortunately this is currently a technically difficult experiment for us to perform. We have however found that we can rescue the cardiac fusion phenotype caused by increased Pdgfra signaling by a sub-phenotypic dose of PI3K inhibitor (Figure 5G-J), further supporting our finding that Pdgfra and PI3K signaling interact to regulate the process of cardiac fusion.
7. Figure 5. Western blots for p-AKT are based on total embryo lysates. Thus, it is expected to find what the authors found related to Pdgfra mutants and hs:pdgfaa. The manuscript would be strengthened if the authors could provide direct evidence of phosphorylated AKT in the myocardial region during cardiac fusion. The authors could use immunofluorescence and confocal imaging.
We thank the reviewer for their suggestion. As we have indicated in our response to
recommendations related to Figures and related conclusions: Query 1, we have
now added results of PH-domain reporter of PI3K activity, which complements our other tissue-specific studies indicating PI3K activity in the myocardium during cardiac fusion.
8. I am a bit conflicted with Supplementary Figure 5C. What each dot represents? A single cell? I suggest the authors read the latest thread about some important statistical considerations:
https://doi.org/10.1083/jcb.202001064
https://rupress.org/jcb/article/219/6/e202001064/151717/SuperPlots-Communicating-reproducibility-and
We thank the reviewer for their suggestion. We have changed our graphs analyzing cardiomyocyte behavior to super plots – which show variation between individual cells as well as variation between embryos.
Reviewer #3 (Recommendations for the authors):
Overall, the manuscript is well written and experiments are well controlled providing sufficient evidence to substantiate most of their conclusions. There are a number of questions that need to be addressed by the authors.
Specific points:
1. What is interesting is that the CPCs have some intrinsic migration that is independent of the endoderm. The finding that Pi3K is required for the cardiac fusion process is not too surprising as the authors showed in their previous work that PDGF signaling is driving this process and PI3K is a well-known transducer of PDGF signaling. An important question remaining is what type of cell migration the CPCs undergo to fuse at the midline. Are the CPCs migrating as individual cells that all respond to the PDGF from the midline or do the CPCs migrate collectively and only a few respond to the PDGF signal? The authors seem to prefer the collective migration of CPCs but it seems that this has not been tested experimentally. Testing this directly would significantly increase the impact of this study. With the myl7:dnPI3K construct the authors seem to have a good tool to test this now. This could be addressed by analysing embryos in which the cardiac field contains a mixture of wt CPCs and CPCs expressing the dnPIK3 for example by cell transplantation experiments in which cells from embryos derived from the myl7:dnPI3K F1 fish are transplanted to wt embryos or by DNA injections resulting in mosaic expression. It would be very interesting to see how CPCs expressing the dnPI3K behave when surrounded by wt cells. If collective migration is indeed driving cardiac fusion one would expect that these dnPI3K cells behave like wt cells.
We appreciate the reviewer’s suggestion. Cardiomyocytes are interconnected to one another via intercellular junctions starting at around 12s (Ye D, et al. 2015, PMID: 26329600), which would support a collective mode of migration. However, the reviewer is correct that this is still an open question, which we hope to address in a future study.
2. In Figure 3 the authors show that migration of CPCs treated with LY is affected. They plotted the CPC velocities in 3E and here it looks as if fast-migrating CPCs are less affected compared to slower-migrating CPCs. Fast migration is mostly seen in the posterior part of the bilateral heart fields, so the question arises whether CPCs along the anterior-posterior axis is affected differently by the LY treatment.
To address this question, we analyzed cardiomyocyte velocity after dividing the bilateral cardiac populations into thirds: Top (anterior), Middle, and bottom (posterior). However, we did not find a significant difference in the cardiomyocyte velocity between DMSO and PI3K-inhibited embryos. This new information is contained (Figure 3 —figure supplement 2F) and on page 13, lines 8-9.
3. In fig3E the authors show the total velocity of the CPCs in all directions. As they conclude at the end that PDGF-PI3K signaling mostly affects the medial cell migration, it would be important to analyse the velocities of the CPCs only in the medial-lateral direction.
We have now analyzed cardiomyocyte velocity in the medial-lateral direction, where we found a significant difference between DMSO (0.3548 -/+ 0.027 microns/min) and PI3Kinhibited embryos (0.1716 -/+ 0.018 microns/min) (Two-tailed T-test: 0.0008). This information is now found in Figure 3 —figure supplement 2E and on page 13, lines 5-9.
4. In Figure 3G the authors show the angles at which the CPCs migrate based on the time lapses. In Figure 3B the tracks show that some cells (mostly located anteriorly) move in a medial-posterior direction while other cells (mostly located in the posterior part) move in an anterior-medial direction. The rose plot however displays only positive angles (medial-anterior direction) and no angles in the medial-posterior direction. Please explain why this was done.
Our goal when calculating the angle of movement was to determine whether cells were directed along the medial-lateral or were directed angularly (along the anterior-posterior axis, as determined by Holtzman et al. 2007). In this analysis we do not distinguish movement in the anterior direction from movement in the posterior direction, which would be problematic for calculating an average. This is in line with how we calculated directionality in the original eLife paper 2017. Unfortunately, this was not clear in our first version, and we thank the reviewer for bringing this to our attention. We have modified the methods to clearly indicate how directionality is calculated (page 31, line 3).
In addition, the angles shown here for wt embryos (average 30 degrees) seem very different from the angles reported for wt embryos in their previous work (Fig6L, average around 60 degrees), which look more like the angles reported here for the LY treated embryos (average around 60 degrees). Please explain this.
We thank the reviewer for their thoughtful consideration of our data. As we indicate in our response to Essential revisions #2 – Overall, our data is consistent with both our previous work and with the work of others (Holtzman et al. 2007). The difference between the two studies may be a result of normalization differences or in slight variations in the developmental stages captured. We now include a discussion of these differences in manuscript (page 18, lines 16-20).
5. In Figure 4 the authors analyse the direction and persistence of cellular protrusion in CPCs. Especially the direction of these protrusions seems affected by LY suggesting that PDGF from the midline may be responsible for this phenomenon. It would therefore be very interesting to test this in their hs:pdgf line by analysing protrusion direction when pdgf is produced by all cells. If PDGF from the midline is responsible for their direction, overexpressing pdgf in all cells should give a similar effect as inhibiting PI3K.
We agree with the reviewer that this is a valuable experiment. Our current manuscript revolves around the role of PI3K signaling in cardiac fusion. As we turn our attention directly to the role of protrusions in cardiac fusion, we think this will be an important experiment to complete.
https://doi.org/10.7554/eLife.85930.sa2Article and author information
Author details
Funding
American Heart Association (18CDA34080195)
- Joshua Bloomekatz
Eunice Kennedy Shriver National Institute of Child Health and Human Development (R15HD108782)
- Joshua Bloomekatz
National Institute of General Medical Sciences (P20GM103460)
- Joshua Bloomekatz
The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank members of the Bloomekatz lab and S Liljegren, B Jones, K Willett, M Jekabsons, Y Qiu for helpful discussions; R Cao and G Roman in the GlyCORE imaging core (NIH-P20GM103460), C Thornton, R Knerr, and P Bolton for animal support as well as C Chang, D Dong, K Kwan, R Parton for providing reagents.
Ethics
All animals were handled according to protocols approved by the University of Mississippi Institutional Animal Care and Use Committee (IACUC) (protocol #21-007), in accordance with the recommendations of the National Institutes of Health (NIH).
Senior Editor
- Didier YR Stainier, Max Planck Institute for Heart and Lung Research, Germany
Reviewing Editor
- Richard P Harvey, Victor Chang Cardiac Research Institute, Australia
Reviewer
- Osvaldo Contreras, Victor Chang Cardiac Research Institute, Australia
Version history
- Preprint posted: January 3, 2023 (view preprint)
- Received: January 13, 2023
- Accepted: November 2, 2023
- Accepted Manuscript published: November 3, 2023 (version 1)
- Version of Record published: November 15, 2023 (version 2)
- Version of Record updated: November 17, 2023 (version 3)
Copyright
© 2023, Shrestha et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 194
- Page views
-
- 37
- Downloads
-
- 0
- Citations
Article citation count generated by polling the highest count across the following sources: Crossref, PubMed Central, Scopus.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Developmental Biology
A growing body of evidence suggests that cell division and basement membrane invasion are mutually exclusive cellular behaviors. How cells switch between proliferative and invasive states is not well understood. Here, we investigated this dichotomy in vivo by examining two cell types in the developing Caenorhabditis elegans somatic gonad that derive from equipotent progenitors, but exhibit distinct cell behaviors: the post-mitotic, invasive anchor cell and the neighboring proliferative, non-invasive ventral uterine (VU) cells. We show that the fates of these cells post-specification are more plastic than previously appreciated and that levels of NHR-67 are important for discriminating between invasive and proliferative behavior. Transcription of NHR-67 is downregulated following post-translational degradation of its direct upstream regulator, HLH-2 (E/Daughterless) in VU cells. In the nuclei of VU cells, residual NHR-67 protein is compartmentalized into discrete punctae that are dynamic over the cell cycle and exhibit liquid-like properties. By screening for proteins that colocalize with NHR-67 punctae, we identified new regulators of uterine cell fate maintenance: homologs of the transcriptional co-repressor Groucho (UNC-37 and LSY-22), as well as the TCF/LEF homolog POP-1. We propose a model in which the association of NHR-67 with the Groucho/TCF complex suppresses the default invasive state in non-invasive cells, which complements transcriptional regulation to add robustness to the proliferative-invasive cellular switch in vivo.
-
- Chromosomes and Gene Expression
- Developmental Biology
Imaging experiments reveal the complex and dynamic nature of the transcriptional hubs associated with Notch signaling.






